Your Location:Home > Products > Cannabis > P-mentha-2,8-dien-1-ol
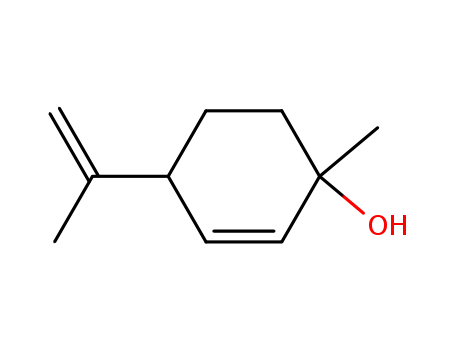

CasNo: 22771-44-4
Molecular Formula: C10H16 O
|
22771-44-4 Name |
|
|
Name |
P-mentha-2,8-dien-1-ol |
|
Synonym |
1-methyl-4-prop-1-en-2-yl-cyclohex-2-en-1-ol;PARA-MENTHA-2,8-DIEN-1-OL;p-Mentha-2,8-dien-1-ol;trans-p-Menthane-2,8-diene-1-ol;D-2,8-P-MENTHADIEN-1-OL;2-Cyclohexen-1-ol, 1-methyl-4-(1-methylethenyl)-;dextro-2,8-para-menthadien-1-ol |
|
22771-44-4 Chemical & Physical Properties |
|
|
Boiling point |
216.9ºC at 760mmHg |
|
Density |
0.947g/cm3 |
|
Molecular Formula |
C10H16O |
|
Molecular Weight |
152.23300 |
|
Flash Point |
87ºC |
|
PSA |
20.23000 |
|
LogP |
2.27970 |
|
Exact Mass |
152.12000 |
|
Vapour Pressure |
0.0294mmHg at 25°C |
|
Index of Refraction |
1.494 |
P-Mentha-2,8-dien-1-ol is a natural product found in Artemisia cina and Artemisia gmelinii with data available, Colorless to very slightly yellow oily liquid; terpiniod aroma.
InChI:InChI=1/C10H16O/c1-8(2)9-4-6-10(3,11)7-5-9/h4,6,9,11H,1,5,7H2,2-3H3
Geraniol (1) and nerol (2) undergo a novel cyclization to cis-p-mentha-2,8-dien-1-ol (4) by reaction with tris(pbromophenyl)ammoniumyl radical cation (3) and the reaction mechanism is discussed.
The main side products were carvone, carveol, 1,2-epoxylimonene diol, perillyl alcohol and p-mentha-2,8-dien-1-ol. An analysis of the time evolution of the reaction, reported in Figure 5, suggests that the main side products, carvone and carveol, are formed via the allylic oxidation route, whereas the diol is formed by the hydration of limonene epoxide; other side products were perillyl alcohol and p-mentha-2,8-dien-1-ol, the latter formed by isomerization of limonene epoxide.
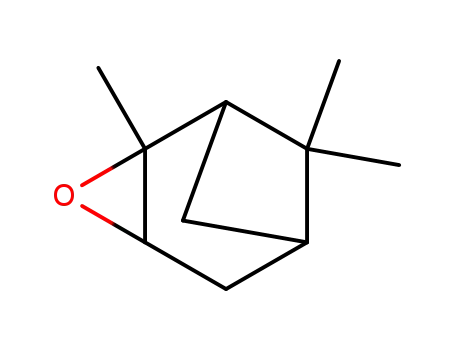
α-pinene epoxide

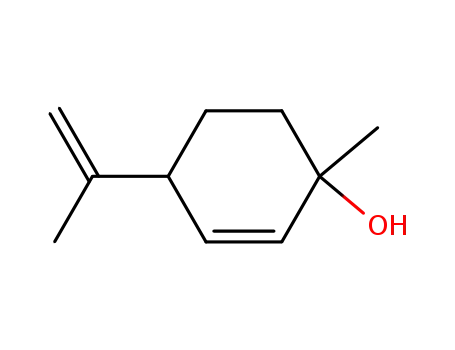
(+)-p-mentha-2,8-dien-1-ol

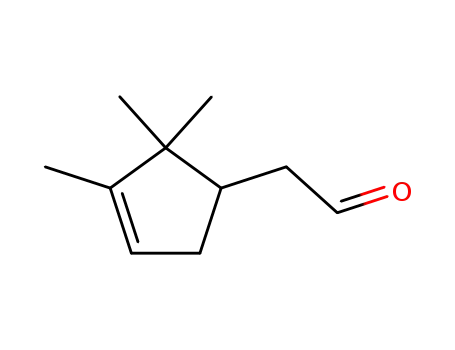
campholenyc aldehyde

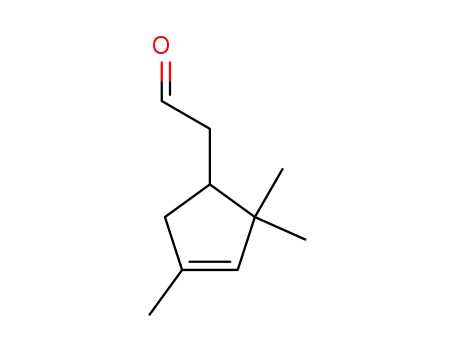
isocampholenic aldehyde

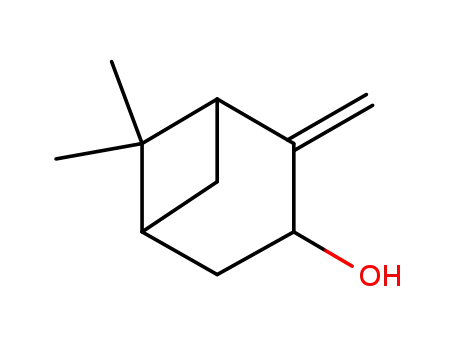
pinocarveol

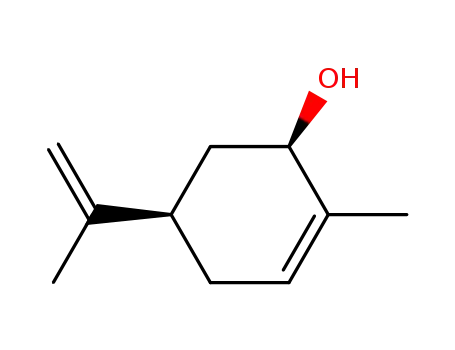
(4R,6R)-carveol

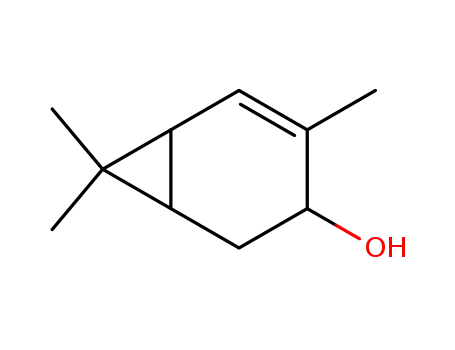
trans-2-caren-4-ol
| Conditions | Yield |
|---|---|
|
With Fe/MCM-41(N2); for 0.166667h;
|

α-pinene epoxide


(+)-p-mentha-2,8-dien-1-ol

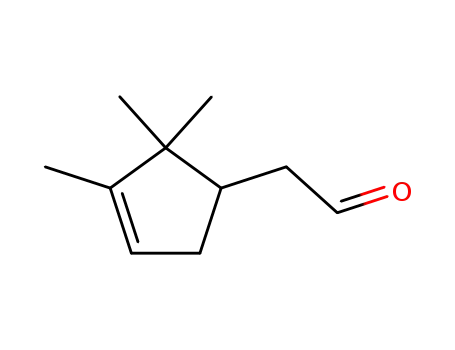
campholenyc aldehyde


isocampholenic aldehyde

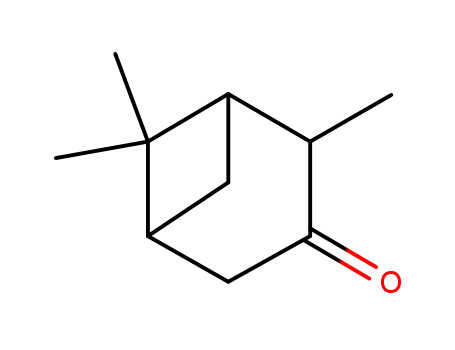
pinocamphone


pinocarveol


(4R,6R)-carveol

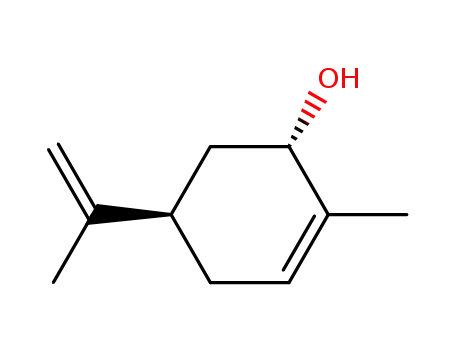
(-)-trans-carveol


trans-2-caren-4-ol
| Conditions | Yield |
|---|---|
|
With Fe/MCM-41(N2); for 0.5h;
|
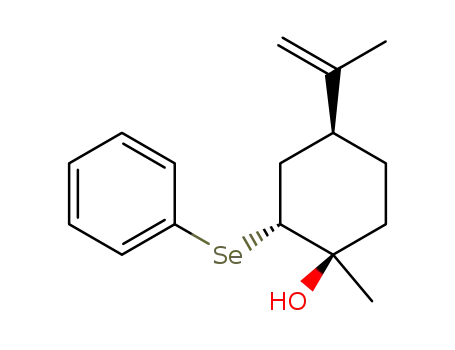
1α-hydroxy-2β-phenylseleno-trans-p-menth-8-ene
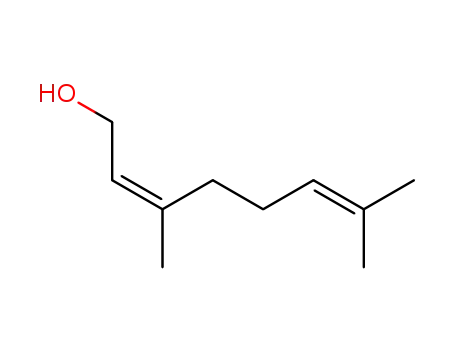
Nerol
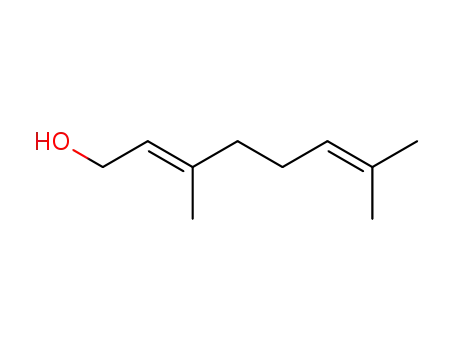
Geraniol
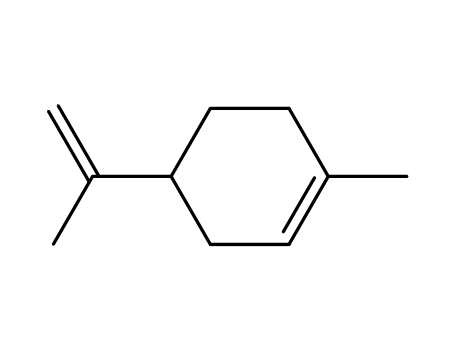
limonene.
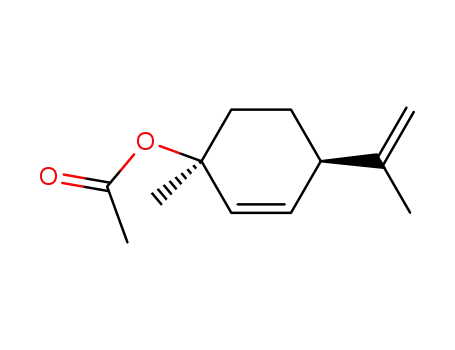
1-acetoxy-trans-p-mentha-2,8-diene
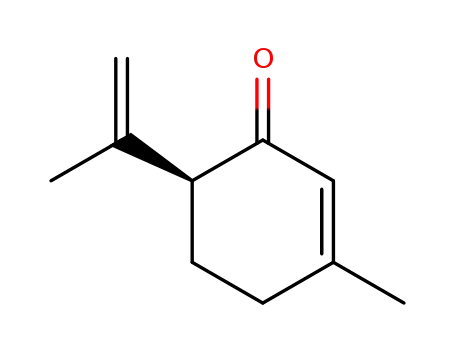
(S)-isopiperitenone
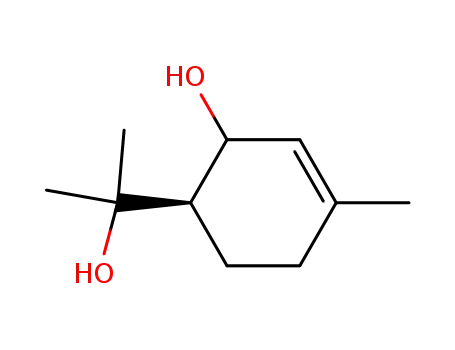
6-(1'-hydroxyisopropyl)-3-methyl-2-cyclohexenol
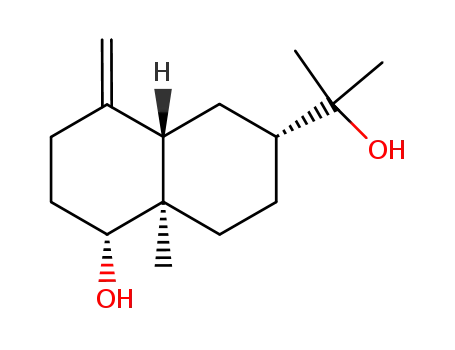
4(15)-eudesmene-1β,11-diol