Your Location:Home > Products > Fine Chemicals > Nonanoyl chloride
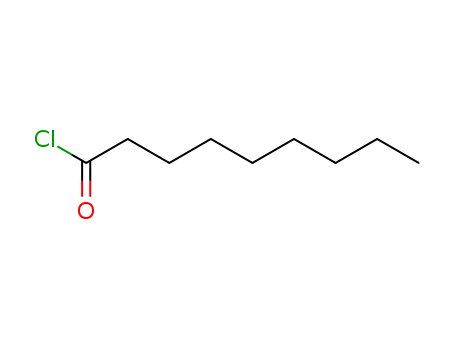

CasNo: 764-85-2
Molecular Formula: C9H17ClO
Appearance: clear slightly yellow liquid
|
764-85-2 Name |
|
|
Name |
Nonanoyl chloride |
|
Synonym |
PELARGONIC ACID CHLORIDE;PELARGONOYL CHLORIDE;PELARGONYL CHLORIDE;N-NONANOYL CHLORIDE;NONYL ACID CHLORIDE;NONANOYL CHLORIDE;NNCL;Nonanoic acid chloride |
|
764-85-2 Chemical & Physical Properties |
|
|
Melting point |
-60.5 °C |
|
Boiling point |
214.7±3.0 °C at 760 mmHg |
|
Density |
1.0±0.1 g/cm3 |
|
Molecular Formula |
C9H17ClO |
|
Molecular Weight |
176.684 |
|
Flash Point |
95.0±0.0 °C |
|
PSA |
17.07000 |
|
LogP |
4.18 |
|
Exact Mass |
176.096786 |
|
Vapour Pressure |
0.2±0.4 mmHg at 25°C |
|
Index of Refraction |
1.437 |
Nonanoyl chloride is clear slightly yellow liquid, which is used for the acylation of amine groups of chitosan to simultaneously improve its material properties and adsorption capacity. It was also used in the synthesis of N-nonanoyl piperonylamide, caged vanilloid, N-(4-hydroxy-3-methoxybenzyl)-N-(2-nitrobenzyl)-nonanoylamide or N-(2-Nitrobenzyl)-N-vanillyl-nonanoylamide, n-benzylnonanamide.
InChI:InChI=1/C9H17ClO/c1-2-3-4-5-6-7-8-9(10)11/h2-8H2,1H3
Bioassay-guided fractionation of the liquid culture broth of Pseudomonas sp. MF381-IODS yielded two new antimicrobial substances, identified as (2E,4E,6E)-9-[((2S,3R)-3-hydroxy-4-{(3E,5E,7RS)-7-hydroxy-4-methylhexadeca-3, 5-dienoyl]amino}-2-methylbutanoyl)amino]nona-2,4,6-trienoic acid and the tetradeca equivalent, named pseudotrienic acids A (1) and B (2), respectively. Two known antimicrobial compounds, the polyketide 2,3-deepoxy-2,3-didehydrorhizoxin (3) and the tryptophan-derived pyrrolnitrin (4), were also identified.
The following esterification using nonanoyl chloride in the presence of triethylamine gave the desire. We report the use of nitrone C–H groups as hydrogen bond donors for binding anions. Acyclic anion receptors with two nitrone moieties were synthesized by condensation reactions between aryl-aldehydes and m-phenylenedihydroxylamines.
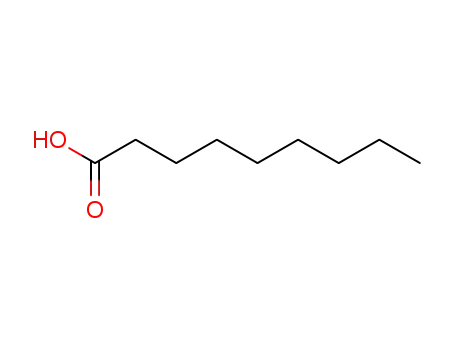
nonanoic acid

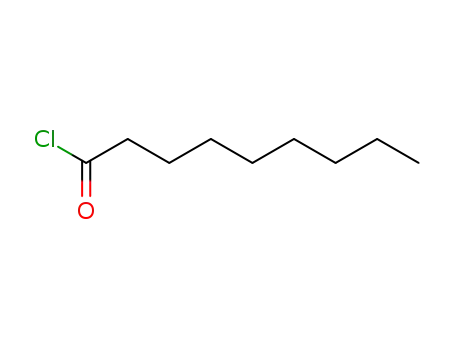
Nonanoyl chloride
| Conditions | Yield |
|---|---|
|
With thionyl chloride; for 4h; Reflux;
|
99% |
|
With zinc(II) chloride; phosphorus trichloride;
|
|
|
With thionyl chloride;
|
|
|
With thionyl chloride;
|
|
|
With oxalyl dichloride;
|
|
|
With oxalyl dichloride; Ambient temperature;
|
|
|
With thionyl chloride; Heating;
|
|
|
With oxalyl dichloride; for 3h;
|
|
|
With thionyl chloride; In benzene; Heating;
|
|
|
With thionyl chloride; for 3h; Heating;
|
|
|
With oxalyl dichloride;
|
|
|
With thionyl chloride; In benzene; Heating;
|
|
|
With thionyl chloride; N,N-dimethyl-formamide; at 50 ℃; for 2.25h;
|
|
|
With phosgene; N,N-dimethyl-formamide; at 20 - 30 ℃;
|
|
|
With hydrogenchloride; phosgene; N,N-dimethyl-formamide; at 20 - 30 ℃;
|
|
|
With oxalyl dichloride; In dichloromethane; at 0 - 20 ℃; for 2h;
|
|
|
With thionyl chloride; In chloroform; at 65 ℃; for 0.5h;
|
|
|
With trichloroacetamide; triphenylphosphine; In dichloromethane; for 1h; Reflux;
|
|
|
With oxalyl dichloride;
|
|
|
With thionyl chloride;
|
|
|
With thionyl chloride; for 2h; Reflux; Cooling with ice;
|
|
|
With thionyl chloride; Reflux;
|
|
|
With thionyl chloride; at 80 ℃; for 16h;
|
|
|
With thionyl chloride; for 4h; Reflux;
|
|
|
With thionyl chloride; In N,N-dimethyl-formamide; at 70 ℃;
|
|
|
With oxalyl dichloride; In dichloromethane; at 20 ℃;
|
|
|
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; at 20 ℃; for 2h;
|
|
|
With thionyl chloride; at 70 ℃; for 4h;
|
|
|
With oxalyl dichloride;
|
|
|
With oxalyl dichloride;
|
|
|
With oxalyl dichloride; In dichloromethane; at 20 ℃; for 0.166667h;
|
|
|
With trichloroacetamide; triphenylphosphine; In dichloromethane; for 1h; Reflux;
|
|
|
With oxalyl dichloride; In dichloromethane; at 0 - 20 ℃; for 2h; Inert atmosphere;
|
|
|
With thionyl chloride; Reflux;
|
|
|
With thionyl chloride; Reflux;
|
|
|
With thionyl chloride; In dichloromethane; N,N-dimethyl acetamide; at 0 - 10 ℃; for 0.5h;
|
|
|
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; at 0 - 20 ℃; for 2h;
|
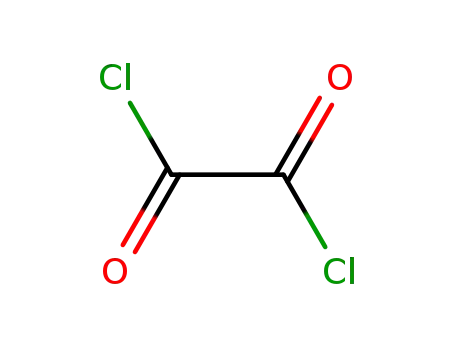
oxalyl dichloride


nonanoic acid


Nonanoyl chloride
| Conditions | Yield |
|---|---|
|
In dichloromethane; N,N-dimethyl-formamide;
|
17.67 g (100%) |
|
In dichloromethane; N,N-dimethyl-formamide;
|
17.67g (100%) |

nonanoic acid

oxalyl dichloride
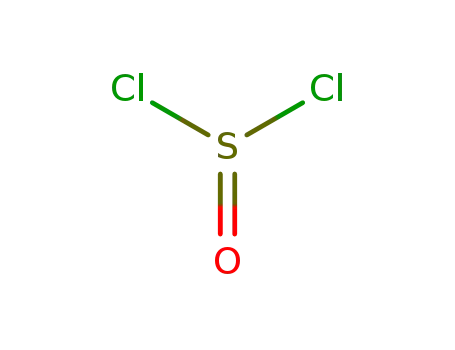
thionyl chloride
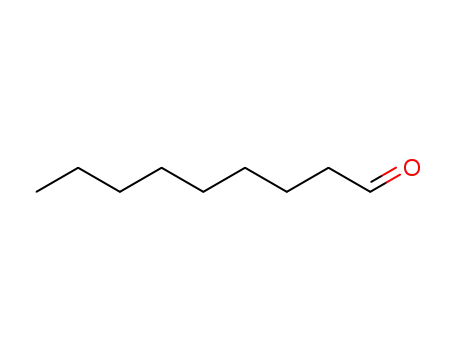
nonan-1-al
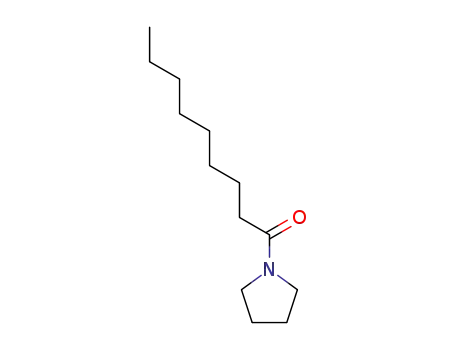
1-(pyrrolidin-1-yl)nonan-1-one
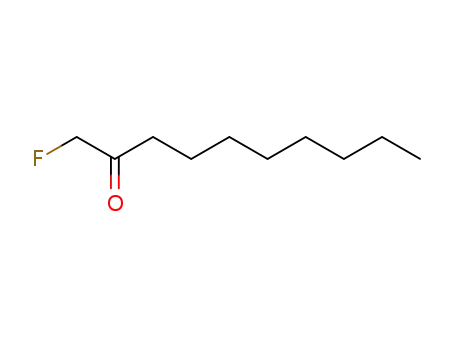
1-fluoro-decan-2-one
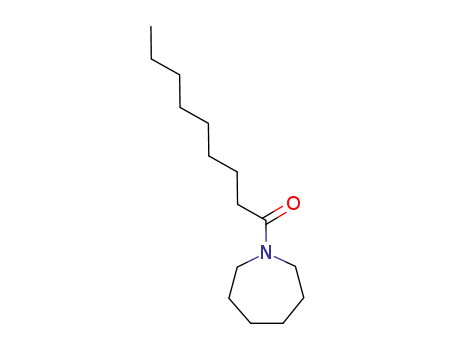
1-nonanoyl-hexahydro-azepine
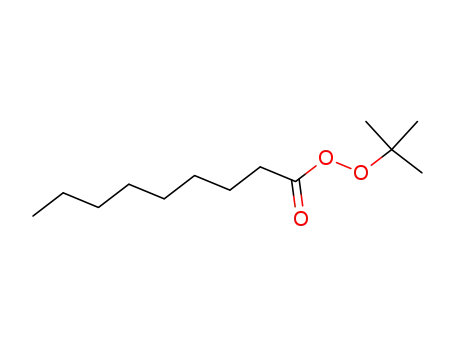
tert-butylperoxy pelargonate