Your Location:Home > Products > Fine Chemicals > N-(tert-Butoxycarbonyl)-4-piperidone
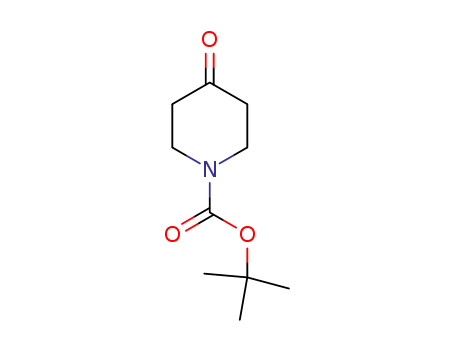

CasNo: 79099-07-3
Molecular Formula: C10H17NO3
Appearance: light yellow powder
|
79099-07-3 Name |
|
|
Name |
N-BOC-4-piperidone |
|
Synonym |
TIMTEC-BB SBB008535;RARECHEM AH CK 0062;TERT-BUTYL 4-OXOPIPERIDINE-1-CARBOXYLATE;TERT-BUTYL 4-OXOTETRAHYDRO-1(2H)-PYRIDINECARBOXYLATE;TERT-BUTYL 4-OXO-1-PIPERIDINECARBOXYLATE;T-BUTYL-4-PIPERIDONE-1-CARBOXYLATE;T-BUTOXYCARBONYL-4-PIPERIDONE;N-BOC-4-PIPERIDINONE |
|
79099-07-3 Chemical & Physical Properties |
|
|
Melting point |
73-77 °C(lit.) |
|
Boiling point |
289.8±33.0 °C at 760 mmHg |
|
Density |
1.1±0.1 g/cm3 |
|
Molecular Formula |
C10H17NO3 |
|
Molecular Weight |
199.247 |
|
Flash Point |
129.1±25.4 °C |
|
PSA |
46.61000 |
|
LogP |
0.38 |
|
Exact Mass |
199.120850 |
|
Vapour Pressure |
0.0±0.6 mmHg at 25°C |
|
Index of Refraction |
1.481 |
|
Storage condition |
Store at 0-5°C |
|
Water Solubility |
slightly soluble |
N-Boc-4-piperidone (NBPO) is a compound useful in organic synthesis used in the preparation of (aminoaryl)(benzyloxy)pyridines as potential antitumor agents. N-Boc-4-piperidone is readily available commercially.
InChI:InChI=1/C10H17NO3/c1-10(2,3)14-9(13)11-6-4-8(12)5-7-11/h4-7H2,1-3H3
The synthetic route for compounds was depicted in Scheme 1. 7-Azaindole 1 reacted with N-Boc-4-piperidone in the refluxed methanol with KOH as the base to afford 3-N-Boc-4-piperidone-7-azaindole 2 with a 90 % yield. The SAR results indicated that substituent R, the N-atom at the 7-position of the 7-azaindole and the double-bond in the 1,2,3,6-tetrahydropyridine skeleton were
A series of 5,6,7,8-tetrahydro-1,6-naphthyridin-2(1H)-one derivatives hydrochloride were obtained using a convenient and mild method from 4-piperidone monohydrate hydrochloride. The newly synthesized compounds and their derivatives were characterized by 1H NMR, 13C NMR, and high-resolution mass spectrometry. Furthermore, cytotoxicity in vitro of the synthesized compounds were screened using MTT or CCK8 assay. The results showed that some of the compounds showed potential antitumor activity. Among of them, compound 10a had effects against tumor cells (MOLM-13), and the half maximal inhibitory concentration value was 76?μmol/L.
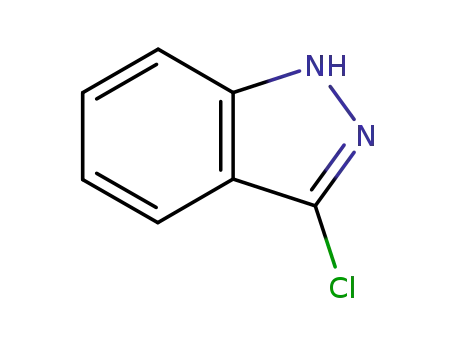
3-chloroindazole

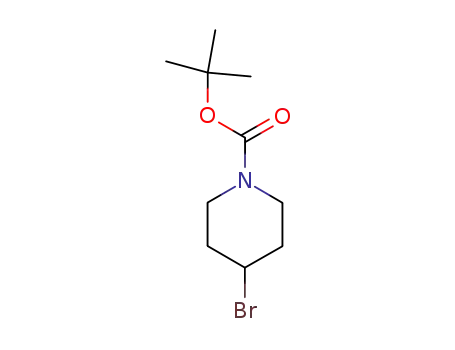
tert-butyl 4-bromo-1-piperidinecarboxylate

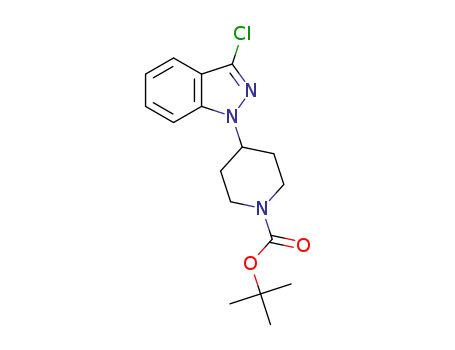
C17H22ClN3O2

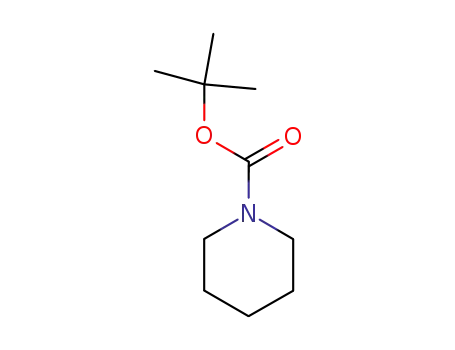
t-butyl piperidinecarboxylate

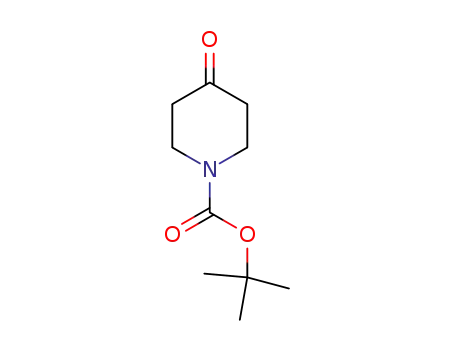
N-tert-butyloxycarbonylpiperidin-4-one

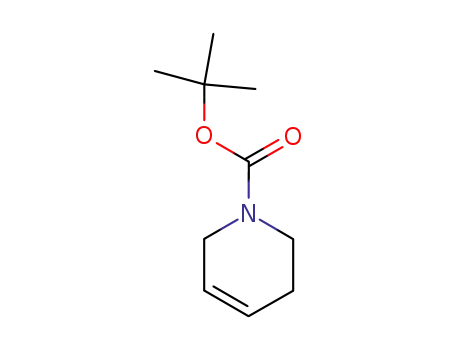
N-Boc-1,2,3,6-tetrahydropyridine
| Conditions | Yield |
|---|---|
|
With copper acetylacetonate; 1,1,1,3,3,3-hexamethyl-2-(trimethylsilyl)-2-trisilanol; [Ir(3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl]phenyl)2(4,4'-bis(trifluoromethyl)bipyridine)]PF6; N,N,N',N'-tetramethylguanidine; In acetonitrile; at 20 ℃; for 18h; regioselective reaction; Irradiation; Sealed tube;
|
23% |
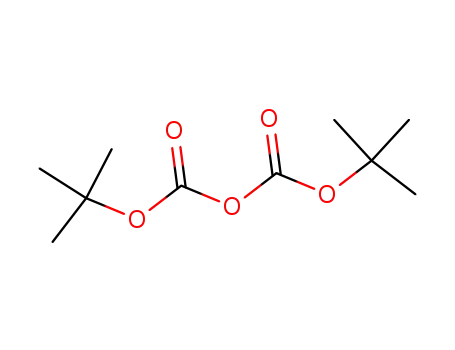
di-tert-butyl dicarbonate

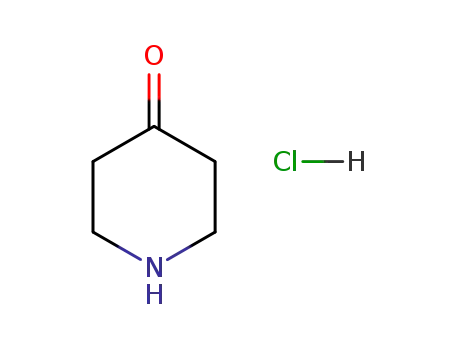
4-piperidone hydrochloride


N-tert-butyloxycarbonylpiperidin-4-one
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; In water; 1) 35 deg C, 1 h; 2) 50 deg C, 2.5 h;
|
100% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 20 ℃; for 16h;
|
100% |
|
di-tert-butyl dicarbonate; 4-piperidone hydrochloride; With triethylamine; dmap; In methanol; at 20 ℃; for 20h;
With hydrogenchloride; In dichloromethane;
|
100% |
|
In 1,4-dioxane; water; at 20 ℃; for 4h;
|
100% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; at 25 ℃; for 6h;
|
98% |
|
With triethylamine; In dichloromethane; at 20 ℃; for 24h;
|
98% |
|
With triethylamine; In 1,4-dioxane; water; at 0 - 20 ℃; Concentration;
|
95% |
|
With sodium hydrogencarbonate; In water; acetone; at 20 ℃; for 24h;
|
94% |
|
With sodium hydrogencarbonate; In 1,4-dioxane; water; at 20 - 70 ℃;
|
93% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 20 ℃; for 2h; Inert atmosphere;
|
93% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 20 ℃; for 16h;
|
93% |
|
With sodium hydroxide; In 1,4-dioxane; water; at 0 ℃; for 0.5h;
|
92.5% |
|
With triethylamine; In dichloromethane; at 0 - 20 ℃; for 0.8h;
|
92% |
|
4-piperidone hydrochloride; With triethylamine; In tetrahydrofuran; for 0.0833333h;
di-tert-butyl dicarbonate; With dmap; In tetrahydrofuran; at 20 ℃; for 12h;
|
91.8% |
|
With triethylamine; In N,N-dimethyl-formamide; for 24h; Ambient temperature;
|
90% |
|
4-piperidone hydrochloride; With sodium hydroxide; In water; at 20 - 30 ℃; for 0.333333h;
di-tert-butyl dicarbonate; In water; at 20 - 30 ℃; for 12h; Concentration; Reagent/catalyst;
|
90% |
|
With 1,4-dioxane; triethylamine; In water; at 20 ℃;
|
89% |
|
With triethylamine; In dichloromethane; at 20 ℃; for 16h;
|
88% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 0 - 25 ℃;
|
87.44% |
|
With sodium carbonate; In 1,4-dioxane; water; at 20 ℃; for 1h;
|
87% |
|
With sodium carbonate; In 1,4-dioxane; water; at 20 ℃; for 1h;
|
87% |
|
With sodium carbonate; In 1,4-dioxane; water; at 20 ℃; for 1h;
|
87% |
|
With sodium carbonate; In 1,4-dioxane; water; at 20 ℃; for 1h;
|
87% |
|
With sodium carbonate; In 1,4-dioxane; at 20 ℃;
|
85% |
|
With sodium carbonate; In water; at 35 - 50 ℃; for 4h;
|
81% |
|
With N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; water; at 20 ℃; for 24h;
|
80.2% |
|
With triethylamine; In dichloromethane; at 20 ℃; for 16h;
|
79% |
|
4-piperidone hydrochloride; With triethylamine; In dichloromethane; at 20 ℃; for 0.5h;
di-tert-butyl dicarbonate; In dichloromethane; at 20 ℃; for 16h;
|
79% |
|
4-piperidone hydrochloride; With sodium hydrogencarbonate; In tetrahydrofuran; water; at 20 ℃;
di-tert-butyl dicarbonate; In tetrahydrofuran; water; at 20 ℃; for 12h;
|
78% |
|
With triethylamine; In methanol; dichloromethane; at 0 - 20 ℃; for 16h;
|
78% |
|
With N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; water; at 20 ℃; for 24h;
|
75% |
|
With N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; at 20 ℃; for 24h;
|
75% |
|
With triethylamine; In methanol; at 20 ℃; for 2h;
|
75% |
|
With triethylamine; In tetrahydrofuran; water; for 2h; Ambient temperature;
|
74.4% |
|
With N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; water; at 20 ℃;
|
73.7% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 10 - 20 ℃;
|
65% |
|
With triethylamine; In N,N-dimethyl-formamide; for 18h; Ambient temperature;
|
63% |
|
With sodium hydrogencarbonate; In water; acetonitrile; at 20 ℃;
|
62% |
|
With sodium hydroxide; In water; at 20 ℃; for 6h;
|
40% |
|
With sodium hydroxide; In tetrahydrofuran; at 20 ℃; for 12h;
|
10.9 g |
|
With sodium hydrogencarbonate; sodium chloride; In tetrahydrofuran; water; at 20 ℃;
|
|
|
With sodium hydroxide; In 1,4-dioxane; water; at 20 ℃;
|
|
|
With potassium carbonate; triethylamine; In chloroform; at 0 - 20 ℃;
|
|
|
With sodium hydrogencarbonate; sodium chloride; In tetrahydrofuran; water; at 20 ℃;
|
|
|
With triethylamine; In methanol; at 20 ℃; for 2h;
|
|
|
4-piperidone hydrochloride; With triethylamine; In dichloromethane; for 0.0833333h;
di-tert-butyl dicarbonate; With dmap; In dichloromethane; at 25 ℃; for 20h;
|
3.9 g |
|
With sodium hydroxide;
|
|
|
With sodium hydroxide; sodium monohydrogen sulfate; In tetrahydrofuran;
|
|
|
With triethylamine; In dichloromethane; at 10 - 30 ℃; for 16.25h;
|
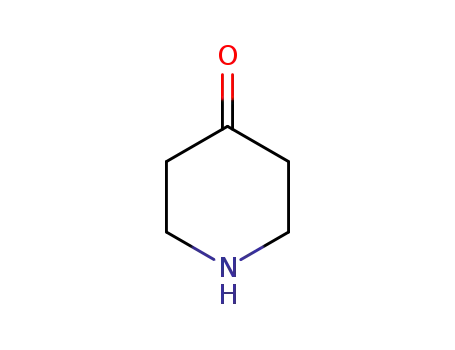
piperidin-4-one

di-tert-butyl dicarbonate

4-piperidone hydrochloride
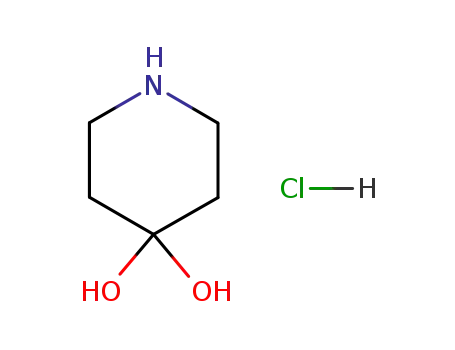
piperidine-4,4-diol hydrochloride
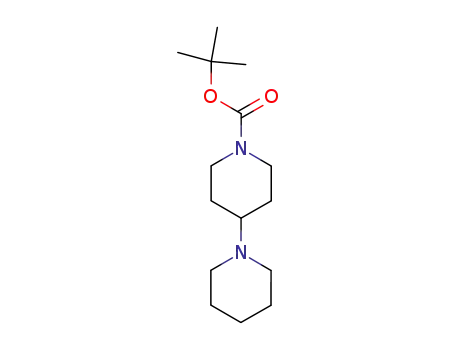
tert-butyl 1,4’-bipiperidine-1’-carboxylate
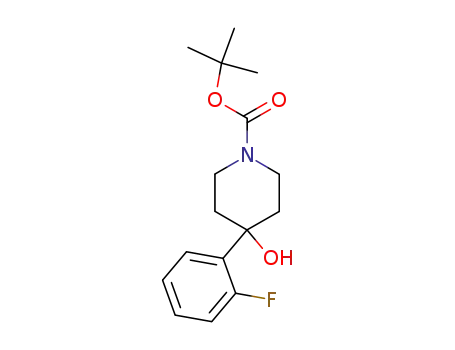
4-(2-fluoro-phenyl)-4-hydroxypiperidine-1-carboxylic acid tert-butyl ester
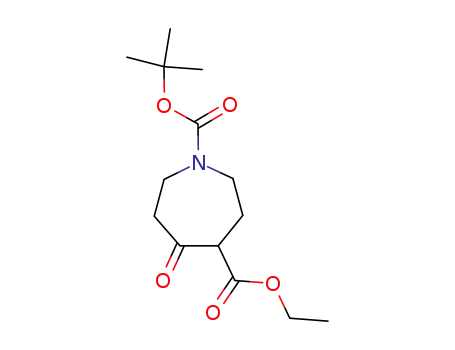
5-oxo-azepane-1,4-dicarboxylic acid 1-tert-butyl ester 4-ethyl ester
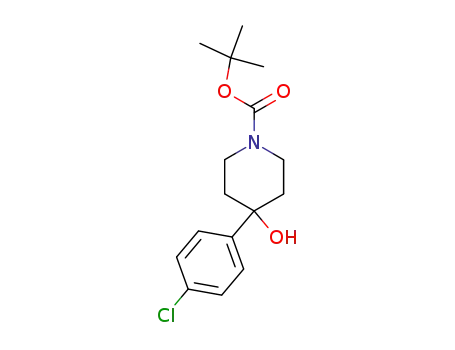
4-(4-chloro-phenyl)-4-hydroxy-piperidine-1-carboxylic acid tert-butyl ester