Your Location:Home > Products > Pharmaceutical > Acetic acid,Dichloroacetate, sodium salt (1:1)
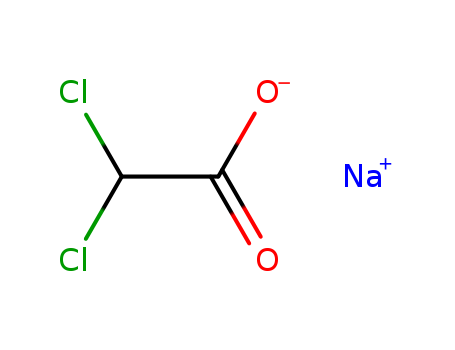

CasNo: 2156-56-1
Molecular Formula: C2HCl2NaO2
Appearance: white powder
|
2156-56-1 Name |
|
|
Name |
Dichloroacetate |
|
Synonym |
dichloroacetate,sodiumsalt;dichloro-aceticacisodiumsalt;dichloroctansodny;Sodiumdichloracetate;NATRIUMDICHLORACETAT;SDA;SODIUM DICHLOROACETATE;erlcyiuanna |
|
2156-56-1 Biological Activity |
|
|
Related Catalog |
Signaling Pathways >> Metabolic Enzyme/Protease >> PDHK Signaling Pathways >> Apoptosis >> Apoptosis Research Areas >> Cancer Signaling Pathways >> Membrane Transporter/Ion Channel >> NKCC |
|
Target |
PDHK; Reactive oxygen species (ROS); Apoptosis; NKCC. |
|
2156-56-1 Chemical & Physical Properties |
|
|
Melting point |
198 °C (dec.)(lit.) |
|
Boiling point |
194ºC at 760mmHg |
|
Molecular Formula |
C2HCl2NaO2 |
|
Molecular Weight |
150.924 |
|
Form |
Powder |
|
PSA |
40.13000 |
|
Exact Mass |
149.925125 |
|
Vapour Pressure |
0.196mmHg at 25°C |
|
Storage condition |
Desiccate at RT |
|
Water Solubility |
soluble in cold water |
Dichloroacetate (DCA) is a xenobiotic of interest to both environmental toxicologists and clinicians. Sodium dichloroacetate is a mitochondrial pyruvate dehydrogenase kinase (PDK) inhibitor that exhibits potent anti-leukemic activity, which represents a potentially novel class of oral antidiabetic agents that reduce blood glucose and lipids without stimulating insulin secretion. Dichloroacetate (DCA) exerts multiple effects on pathways of intermediary metabolism. Recent studies had found that DCA acts as a potential vasoprotective agent by inhibiting PDK2 and reducing coronary endarterium proliferation. Further, DCA promotes brain regeneration after cerebral ischemia, which indicates that DCA might play an important role in VD.
InChI:InChI=1/C2H2Cl2O2.Na/c3-1(4)2(5)6;/h1H,(H,5,6);/q;+1/p-1
Dichloroacetate (DCA), a PDK1 inhibitor, was used in vivo to shift intrasynovial tendon ATP production from glycolysis to OXPHOS. Oral DCA administration reduced serum lactate concentration and increased acetyl-CoA content in repaired intrasynovial tendons and led to reduced TLR4 and IL1B and increased IGF1, SCX, and TGFB3 expressions in treated intrasynovial tendons compared to controls.
The bidentate coordination mode of the carboxylato ligands in 1–5 was unambiguously ascertained by IR and NMR spectroscopy, moreover the solid state structure of 1 was elucidated by single crystal X-ray diffraction. Complexes 1–3 experience rapid and quantitative dissociation of the carboxylato anion in DMSO/water/NaCl mixtures, mainly converting into [RuCl2(DMSO)(η6-p-cymene)], 7.
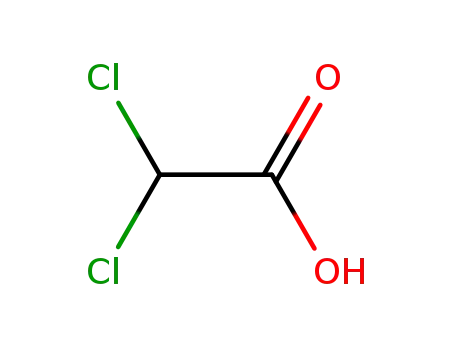
dichloro-acetic acid

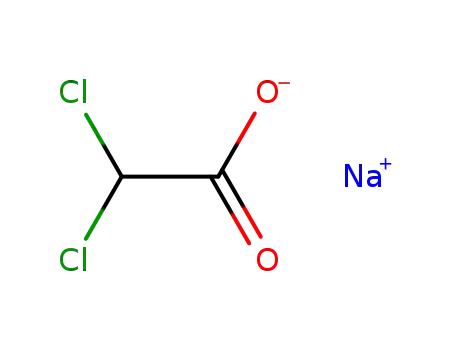
sodium dichloroacetate
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In water; at 20 ℃; for 2h;
|
96% |
|
With sodium hydrogencarbonate; In N,N-dimethyl-formamide;
|
diethyl ether


sodium dichloroacetate

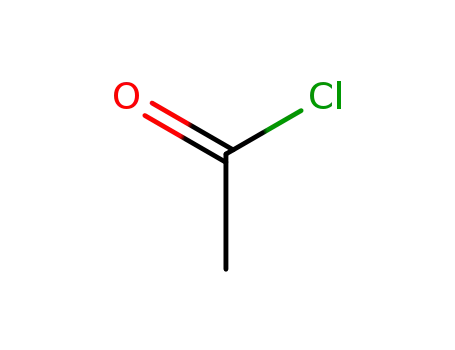
acetyl chloride

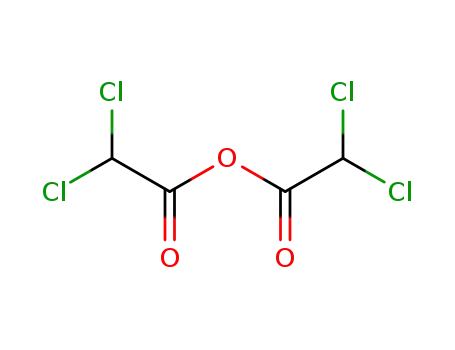
dichloroacetic anhydride
| Conditions | Yield |
|---|---|
|
|

dichloro-acetic acid
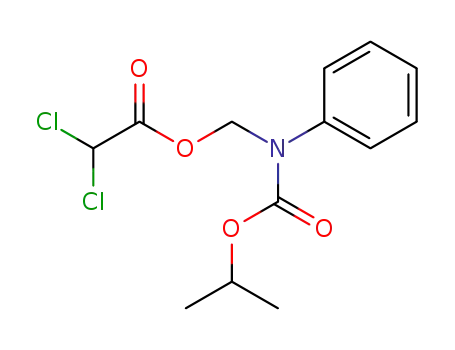
Dichloro-acetic acid (isopropoxycarbonyl-phenyl-amino)-methyl ester
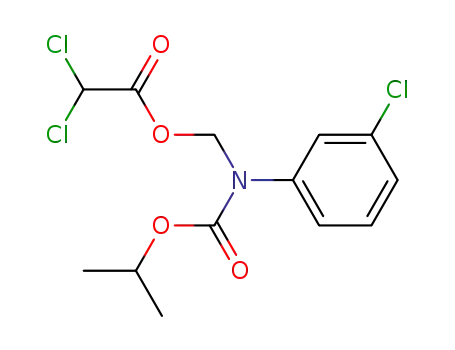
Dichloro-acetic acid [(3-chloro-phenyl)-isopropoxycarbonyl-amino]-methyl ester
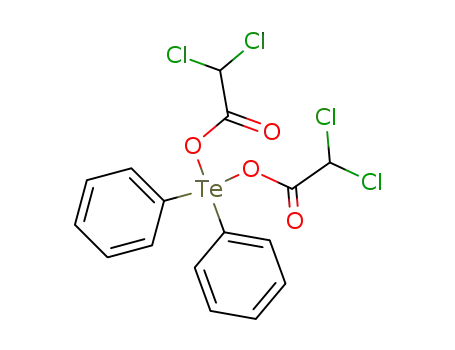
diphenyltellurium(IV) bis(dichloroacetate)
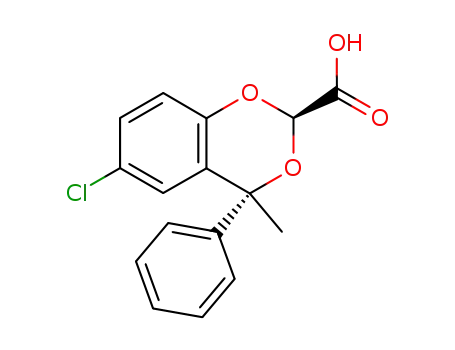
acide