Your Location:Home > Products > 4-Pyridinecarboxaldehyde,2-bromo-


CasNo: 118289-17-1
Molecular Formula: C6H4BrNO
|
118289-17-1 Name |
|
|
Name |
2-Bromo-4-pyridinecarboxaldehyde |
|
Synonym |
2-BROMO-4-PYRIDINECARBOXALDEHYDE;2-BROMO-4-FORMYLPYRIDINE;2-BROMOPYRIDINE-4-CARBALDEHYDE;2-BROMOPYRIDINE-4-CARBOXALDEHYDE;2-bromo-4-pyridinecarboxaldehydecarboxaldehyde;2-Bromopyridine-4-carbaldehyde ,97%;2-Bromoisonicotinaldehyde;2-Bromo-4-formylpyridine, 2-Bromoisonicotinaldehyde |
|
118289-17-1 Chemical & Physical Properties |
|
|
Melting point |
52-56°C |
|
Boiling point |
272.9±20.0 °C at 760 mmHg |
|
Density |
1.7±0.1 g/cm3 |
|
Molecular Formula |
C6H4BrNO |
|
Molecular Weight |
186.006 |
|
Flash Point |
118.8±21.8 °C |
|
PSA |
29.96000 |
|
LogP |
1.34 |
|
Exact Mass |
184.947617 |
|
Vapour Pressure |
0.0±0.6 mmHg at 25°C |
|
Index of Refraction |
1.619 |
InChI:InChI=1/C6H4BrNO/c7-6-3-5(4-9)1-2-8-6/h1-4H
A simple, efficient, and general two-step synthesis to bromo-pyridine carbaldehyde scaffolds is described. Bromo-pyridine carbaldehyde scaffolds 1-7 were obtained in good overall yield. Bromo-dibromomethyl-pyridine intermediates have been isolated and characterized.
The invention relates to aminotriazole derivatives of formula (I), wherein A, E, R1 and R2 are as defined in the description, their preparation and their use as pharmaceutically active compounds. The compounds are useful for the prevention or treatment of diseases, which respond to the modulation of the ALX receptor such as inflammatory diseases.
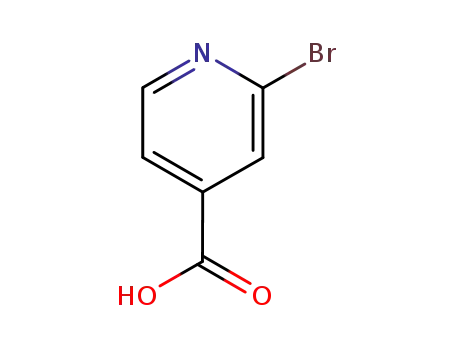
2-bromoisonicotinic acid

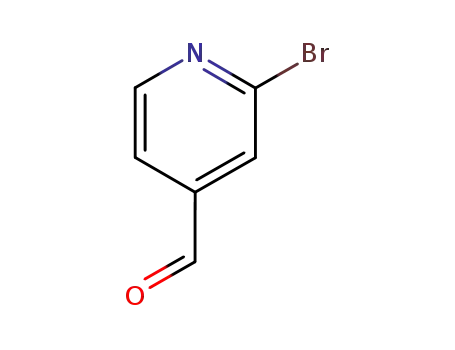
2-bromopyridine-4-carboxaldehyde
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 1) NEt3, ethyl chloroformate, 2) LiAlH4 / 1) benzene, rt, 1 h, 2) THF, -78 deg C, 30 min
2: 88 percent / DMSO, N,N'-dicyclohexylcarbodiimide, H3PO4 / 1.5 h / Ambient temperature
With lithium aluminium tetrahydride; phosphoric acid; chloroformic acid ethyl ester; dimethyl sulfoxide; triethylamine; dicyclohexyl-carbodiimide;
|
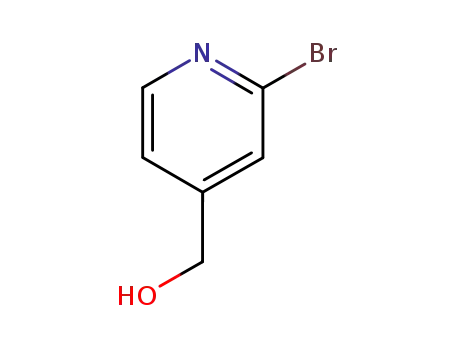
(2-bromopyridin-4-yl)methanol


2-bromopyridine-4-carboxaldehyde
| Conditions | Yield |
|---|---|
|
With phosphoric acid; dimethyl sulfoxide; dicyclohexyl-carbodiimide; for 1.5h; Ambient temperature;
|
88% |
|
(2-bromopyridin-4-yl)methanol; With oxalyl dichloride; dimethyl sulfoxide; In dichloromethane; at -60 ℃; for 0.166667h; Inert atmosphere;
With triethylamine; In dichloromethane; at -60 - 20 ℃; Inert atmosphere;
|
62% |
|
With manganese(IV) oxide; In dichloromethane; at 20 ℃; for 54h;
|

(2-bromopyridin-4-yl)methanol
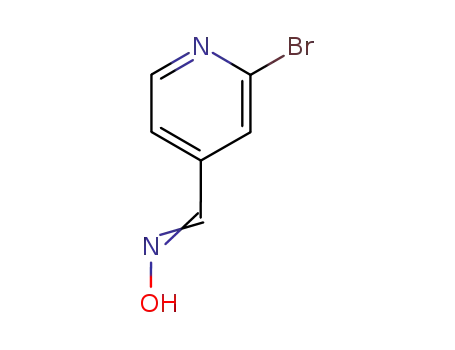
2-bromo-pyridine-4-carbaldehyde oxime
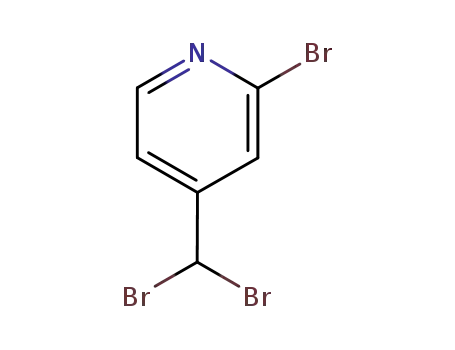
2-bromo-4-(dibromomethyl)pyridine
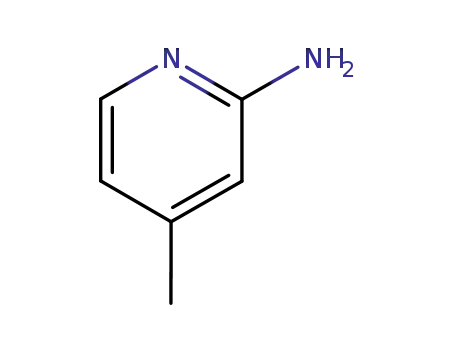
2-Amino-4-methylpyridine
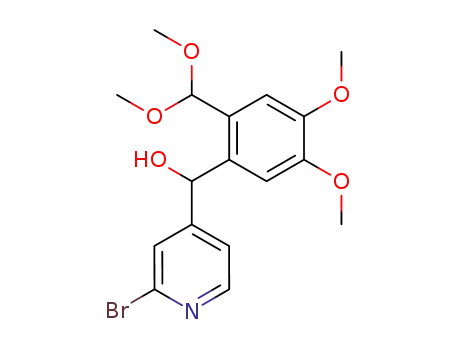
3,4-dimethoxy-6-(2-bromo-4-pyridyl)(hydroxy)methylbenzaldehyde dimethylacetal
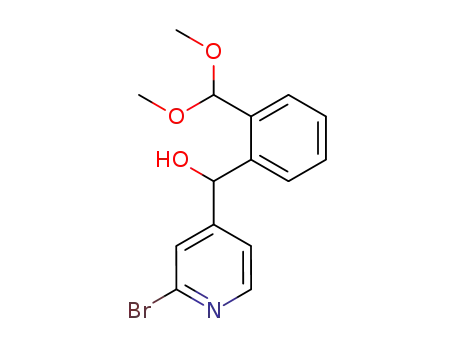
(2-bromo-pyridin-4-yl)-(2-dimethoxymethyl-phenyl)-methanol
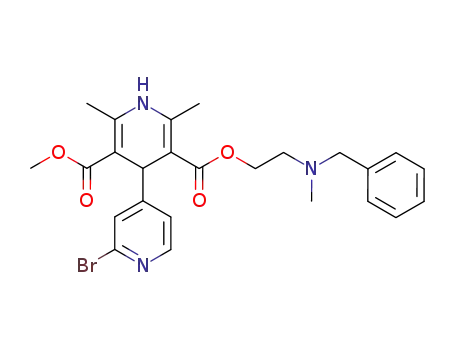
2'-Bromo-2,6-dimethyl-1,4-dihydro-[4,4']bipyridinyl-3,5-dicarboxylic acid 5-[2-(benzyl-methyl-amino)-ethyl] ester 3-methyl ester
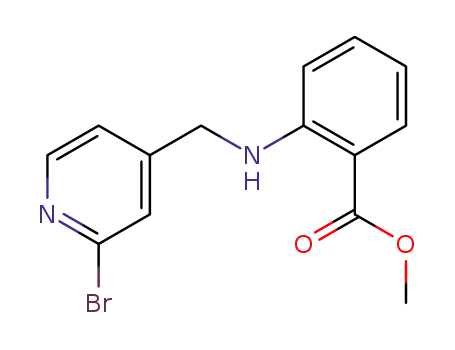
2-[(2-bromo-pyridin-4-ylmethyl)-amino]-benzoic acid methyl ester