Your Location:Home > Products > Pharmaceutical > 2-Bromoisonicotinic acid


CasNo: 66572-56-3
Molecular Formula: C6H4BrNO2
Appearance: white solid
|
66572-56-3 Name |
|
|
Name |
2-Bromopyridine-4-carboxylic acid |
|
Synonym |
IFLAB-BB F1926-0035;2-BROMOPYRIDINE-4-CARBOXYLIC ACID;2-BROMOISONICOTINIC ACID;2-BROMO-4-PYRIDINECARBOXYLIC ACID;4-PYRIDINECARBOXYLIC ACID, 2-BROMO-;RARECHEM AL BE 1524;2-bromo-4-picolinic acid;2-BROMO-4-PYRIDINECARBOXYLIC ACID / 2-BROMOISONICOTINIC ACID |
|
66572-56-3 Chemical & Physical Properties |
|
|
Melting point |
229-231°C |
|
Boiling point |
447.2±30.0 °C at 760 mmHg |
|
Density |
1.8±0.1 g/cm3 |
|
Molecular Formula |
C6H4BrNO2 |
|
Molecular Weight |
202.005 |
|
Flash Point |
224.3±24.6 °C |
|
PSA |
50.19000 |
|
LogP |
1.39 |
|
Exact Mass |
200.942535 |
|
Vapour Pressure |
0.0±1.1 mmHg at 25°C |
|
Index of Refraction |
1.617 |
|
Storage condition |
Keep Cold |
Pharmaceutical Intermediates 66572-56-3 is White to light brown powder. 2-Bromoisonicotinic Acid is a useful research chemical for organic synthesis and other chemical processes.
InChI:InChI=1/C6H4BrNO2/c7-5-3-4(6(9)10)1-2-8-5/h1-3H,(H,9,10)/p-1
In this report, we have designed and synthesized a novel switching molecule whose fluorescence can be switched via dynamic conformational change between expanded and shrunk states induced by metal complexation and decomplexation. UV-vis and fluorescence titration studies revealed that metal complexation of the bipyridine units with Zn2+ ions induced the dynamic structural change of the molecular shape and simultaneous enhancement of fluorescence of the Zn2+-porphyrin fluorophore.
In this work three non-symmetric phenylpyridine type ligands, L1, L2 and L3, were designed, and their corresponding Iridium complexes, C1, C2 and C3, synthetized, in order to understand the effect of ligand asymmetry on the properties of the complexes, and to explore their potentiality in devices. The energy of the HOMO and LUMO correlated well with the experimental electrochemical data, and supported the understanding of the processes observed.
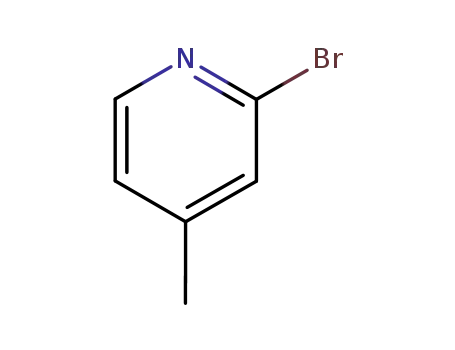
2-Bromo-4-picoline

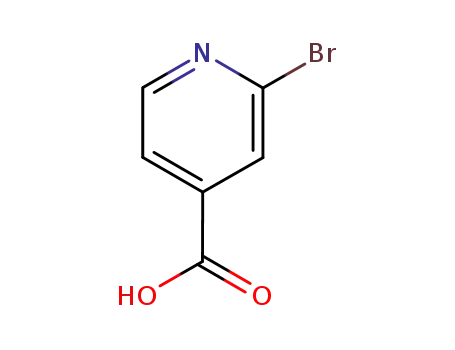
2-bromoisonicotinic acid
| Conditions | Yield |
|---|---|
|
With potassium dichromate; sulfuric acid; at 20 - 50 ℃; for 2h;
|
88% |
|
With potassium dichromate; sulfuric acid; at 20 ℃; for 3h;
|
88% |
|
With potassium permanganate; In water; Heating;
|
48% |
|
2-Bromo-4-picoline; With potassium permanganate; In pyridine; water; at 95 ℃; for 96h;
With hydrogenchloride; In water;
|
47% |
|
With potassium permanganate; In water; at 110 ℃; for 5h;
|
36% |
|
With potassium permanganate; In water; for 7h; Heating;
|
35% |
|
2-Bromo-4-picoline; With potassium permanganate; water; for 5h; Heating / reflux;
With hydrogenchloride; In water; pH=~ 3;
|
34% |
|
With potassium permanganate; In water; for 5h; Heating;
|
31% |
|
With potassium permanganate; In water; Reflux;
|
20.3% |
|
With potassium permanganate; Heating;
|
|
|
2-Bromo-4-picoline; With potassium permanganate; In water; for 3h; Heating / reflux;
With hydrogenchloride; pH=2;
|
|
|
With potassium dichromate; In sulfuric acid;
|
30.0 g (88%) |
|
With potassium permanganate; In water; for 2h; Heating / reflux;
|
|
|
With potassium permanganate;
|
|
|
2-Bromo-4-picoline; With sulfuric acid; at 0 ℃; for 0.5h;
With potassium dichromate; at 0 - 20 ℃; for 11.5h;
|
|
|
2-Bromo-4-picoline; With sulfuric acid; at 0 ℃; for 0.5h;
With potassium dichromate; at 0 - 20 ℃; for 12h;
|
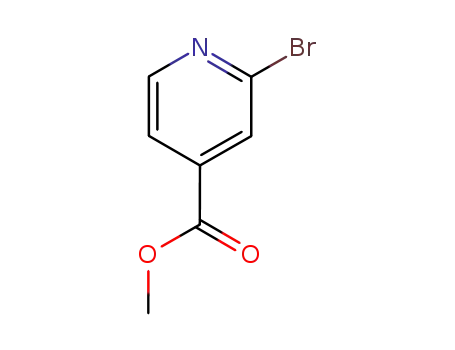
methyl 2-bromoisonicotinate


2-bromoisonicotinic acid
| Conditions | Yield |
|---|---|
|
With lithium hydroxide monohydrate; In tetrahydrofuran; water; at 20 ℃; for 16h;
|
0.246g |

2-Bromo-4-picoline
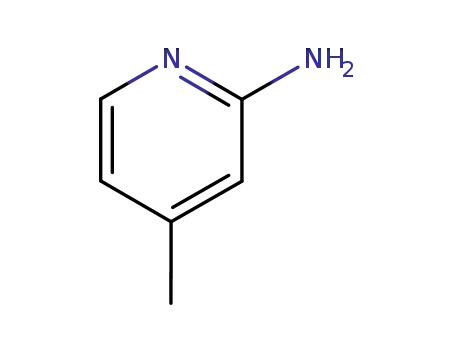
2-Amino-4-methylpyridine
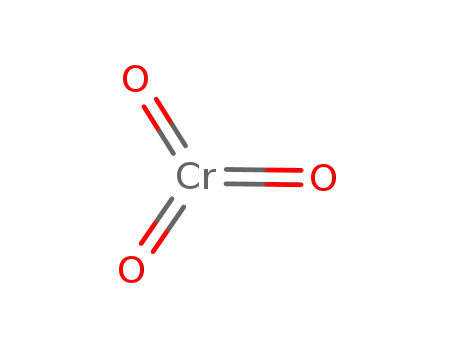
chromium(VI) oxide
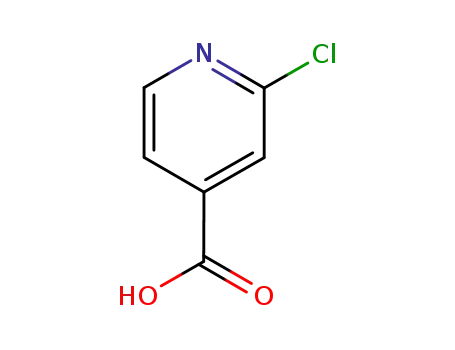
2-chloroisonicotinic acid,

methyl 2-bromoisonicotinate
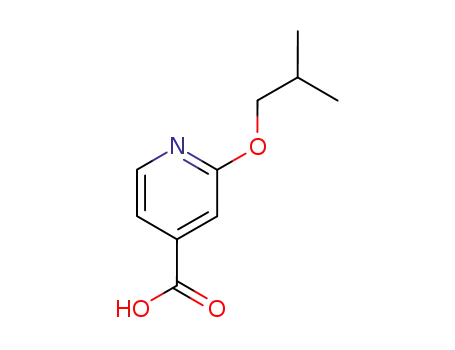
2-isobutoxy-isonicotinic acid
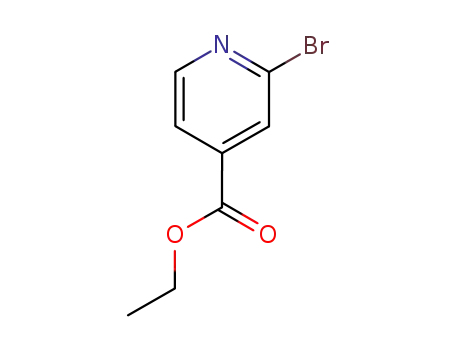
2-bromo-4-carboxylic acid ethyl pyridine
(2-bromopyridin-4-yl)methanol