Your Location:Home > Products > Fine Chemicals > 9,10-Dibromoanthracene
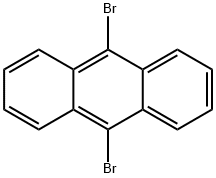

CasNo: 523-27-3
Molecular Formula: C14H8Br2
Appearance: yellow to yellow-green fluffy powder
|
523-27-3 Name |
|
|
Name |
9,10-Dibromoanthracene |
|
Synonym |
9,10-Dibromoanthracene99%;9,I O-Dibromanthracen;9,10-DIBROMO ANTHRACENEN;9,10-Dibromoanthracene,96%;Anthracene,9,10-dibromo-;ms-Dibromoanthracene;9,10-DIBROMOANTHRACENE;9,10-DIRBOMOANTHRACENE |
|
523-27-3 Chemical & Physical Properties |
|
|
Melting point |
220-225 °C |
|
Boiling point |
427.1±18.0 °C at 760 mmHg |
|
Density |
1.8±0.1 g/cm3 |
|
Molecular Formula |
C14H8Br2 |
|
Molecular Weight |
336.021 |
|
Flash Point |
248.1±20.5 °C |
|
LogP |
6.29 |
|
Exact Mass |
333.899261 |
|
Vapour Pressure |
0.0±1.0 mmHg at 25°C |
|
Index of Refraction |
1.749 |
9,10-Dibromoanthracene is Yellow to yellow-green fluffy powder and fluorescent single crystals of 9,10-dibromoanthracene are elastically bendable and stretchable. Melting point 226°C. It can be sublimated and oxidized to generate anthraquinone. Soluble in hot benzene and hot toluene, slightly soluble in alcohol, ether and cold benzene, insoluble in water. 9,10-Dibromoanthracene is an important raw material and intermediate used in organic synthesis, pharmaceuticals and agrochemicals.
InChI:InChI=1/C14H8Br2/c15-13-9-5-1-2-6-10(9)14(16)12-8-4-3-7-11(12)13/h1-8H
An efficient synthesis is described for hexabromoanthracenes 3 and 4 by direct bromination of 9,10-dibromoanthrecene 2. Bromoanthracenes and isomeric arene oxides constitute valuable precursors for the preparation of functionalized substituted anthracene derivatives that are difficult to prepare by other routes.
A mild, metal-free bromination method of arenes has been developed using the combination of bis(trifluoroacetoxy)iodobencene and trimethylsilyl bromide. Density functional theory calculations indicate a stepwise mechanism involving a double bromine addition followed by a type II dyotropic reaction with concomitant re-aromatization of the six-membered ring.
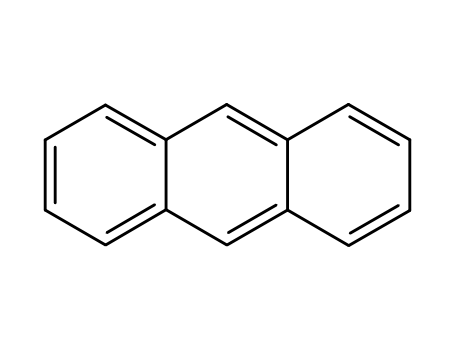
anthracene

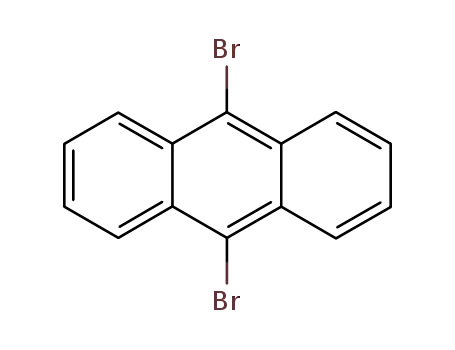
9,10-Dibromoanthracene
| Conditions | Yield |
|---|---|
|
With N-Bromosuccinimide; lithium perchlorate; silica gel; In dichloromethane; at 20 ℃; for 0.0833333h;
|
100% |
|
With oxygen; 1,1-dibromomethane; at 20 ℃; for 2h;
|
99% |
|
With N-Bromosuccinimide; lithium perchlorate; silica gel; In dichloromethane; for 0.5h;
|
99% |
|
With bromine; In chloroform; at 20 ℃; for 4h; Inert atmosphere;
|
98% |
|
With bromine; In chloroform; at 20 ℃; for 4h;
|
98% |
|
With bromine; In dichloromethane; at 0 ℃; for 1h;
|
96% |
|
With bromine; In dichloromethane; at 0 ℃;
|
96% |
|
With bromine; In chloroform; for 4h;
|
96% |
|
With dimethylbromosulphonium bromide; In dichloromethane; at 20 ℃; for 0.5h; Solvent; Concentration; Time;
|
96% |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene hydrobromide-perbromide; In acetic acid; for 0.5h; Heating;
|
95% |
|
With bromine; In chloroform; at 20 ℃; for 5h; Inert atmosphere;
|
95% |
|
With bromine; In acetic acid; at 20 ℃; for 0.5h;
|
94% |
|
With tetrabutylammomium bromide; dihydrogen peroxide; vanadia; In water; acetonitrile; at 5 ℃; for 1h;
|
93% |
|
With potassium bromide; In dichloromethane; water; acetic acid; at 45 ℃; for 5.25h; Reagent/catalyst;
|
93% |
|
With bromine; In chloroform; at 20 ℃; for 4h;
|
92% |
|
With N-Bromosuccinimide; silver hexafluoroantimonate; 1-methylthiotriptycene; In 1,2-dichloro-ethane; at 20 ℃; for 4h; Inert atmosphere; Schlenk technique;
|
92% |
|
anthracene; With bromine; acetic acid; at 20 ℃; for 2h;
With PS-1,5-COD; In tetrahydrofuran; at 20 ℃; for 1h;
|
90% |
|
With bromine; In chloroform; at 20 ℃; for 4h; Inert atmosphere; Darkness;
|
88% |
|
With pyridinium bromochromate; In acetic acid; for 0.5h; water-bath;
|
84% |
|
With N-Bromosuccinimide; In chloroform; acetic acid; at 40 ℃; for 2h;
|
75% |
|
With bromine; In chloroform; at 20 ℃; for 4h;
|
74% |
|
With bromine; In dichloromethane; at 0 ℃;
|
70% |
|
With nitrodibromoacetonitrile; In dichloromethane; for 3h;
|
65% |
|
With bromine; In dichloromethane; at 0 - 20 ℃; for 3.25h; Inert atmosphere;
|
65% |
|
With aluminum oxide; N-Bromosuccinimide; at 45 ℃; for 0.333333h;
|
63% |
|
With tetra-N-butylammonium tribromide; In acetic acid; for 0.5h; Ambient temperature;
|
55% |
|
With sodium bismuthate; zinc dibromide; In acetic acid; at 20 ℃; for 3h;
|
52% |
|
With ammonium metavanadate; dihydrogen peroxide; potassium bromide; In water; acetonitrile; for 48h; Ambient temperature; pH 5;
|
50% |
|
With tetrachloromethane; bromine; Erhitzen zum Sieden; Reinigung durch Behandeln mit CCl4;
|
|
|
With 1,4-dioxane; bromine;
|
|
|
With sulfuric acid; potassium bromide; Erwaermen des Reaktionsprodukts ohne Loesungsmittel oder Behandeln des Reaktionsprodukts mit Zink-Pulver und Eisessig;
|
|
|
With 1,4-dioxane; bromine;
|
|
|
With carbon disulfide; bromine;
|
|
|
With bromine; silica gel; In tetrachloromethane; at 20 ℃; for 0.0833333h;
|
86 % Chromat. |
|
Multi-step reaction with 2 steps
1: benzene; PBr5
2: benzene; PBr5
With phosphorus pentabromide; benzene;
|
|
|
With bromine;
|
|
|
With bromine;
|
|
|
With bromine; In dichloromethane;
|
|
|
With tetra-N-butylammonium tribromide; acetic acid; at 20 ℃; for 0.5h;
|
|
|
With N-Bromosuccinimide; tri-n-butylphosphine sulfide; In chloroform-d1; at 20 ℃; for 0.416667h; Solvent; Catalytic behavior;
|
99 %Spectr. |
|
With bromine; In dichloromethane; at 0 ℃; for 0.5h;
|
99 %Spectr. |
|
With bromine; acetic acid;
|
|
|
With bromine; In chloroform; at 20 ℃; for 5h; Inert atmosphere;
|
|
|
Multi-step reaction with 2 steps
1: bis(trifluoroacetoxy)iodobencene; trimethylsilyl bromide / dichloromethane / 20 °C / Inert atmosphere
2: bis(trifluoroacetoxy)iodobencene; trimethylsilyl bromide / dichloromethane / 20 °C / Inert atmosphere
With bis(trifluoroacetoxy)iodobencene; trimethylsilyl bromide; In dichloromethane;
|
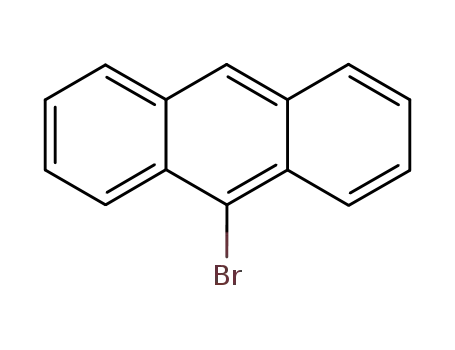
9-Bromoanthracene


9,10-Dibromoanthracene
| Conditions | Yield |
|---|---|
|
With N-Bromosuccinimide; C5H13NO3S; In n-heptane; at 25 ℃; for 19h; Reagent/catalyst; Darkness;
|
85% |
|
With bis(trifluoroacetoxy)iodobencene; trimethylsilyl bromide; In dichloromethane; at 20 ℃; Inert atmosphere;
|
83% |
|
With phosphorus pentabromide; benzene;
|
|
|
With nitric acid; acetic acid;
|
|
|
With 2,6-di-tert-butyl-pyridine; Bromoform; In acetonitrile; platinum mesh electrodes, H cell containing 0.1 M Bu4NPF6, constant current 10 mA;
|
57 % Chromat. |
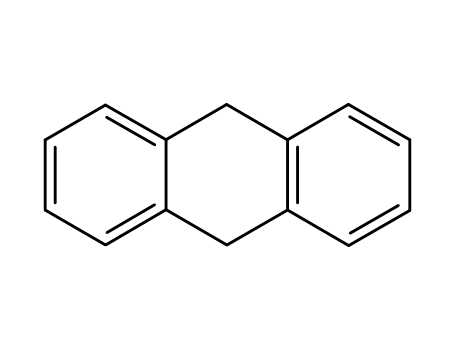
9,10-dihydroanthracene
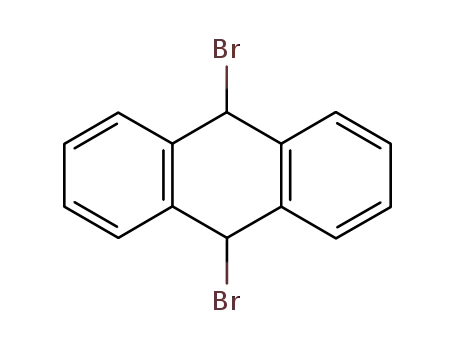
9,19-Dibromo-9,10-dihydroanthracene
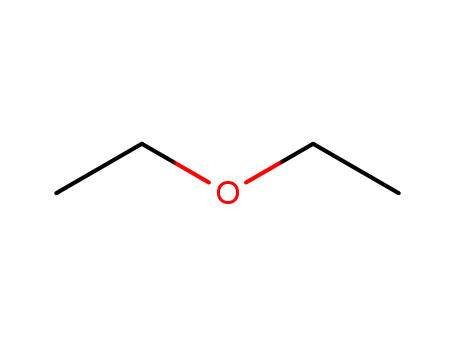
diethyl ether
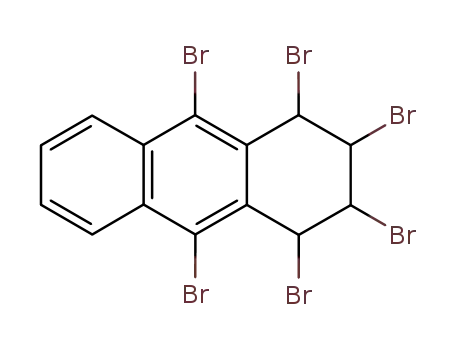
1,2,3,4,9,10-hexabromo-1,2,3,4-tetrahydro-anthracene
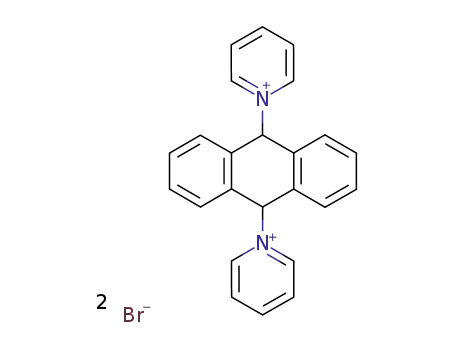
1,1'-(9,10-dihydro-anthracene-9,10-diyl)-bis-pyridinium; dibromide
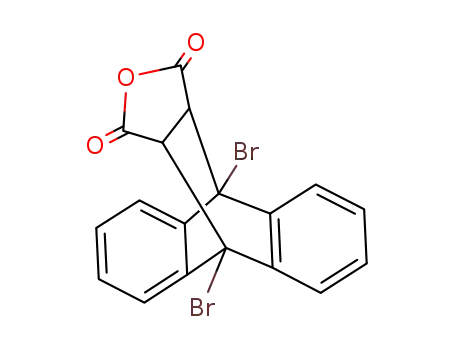
9,10-Ethano-9,10-dibrom-9,10-dihydro-anthracen-11,12-dicarbonsaeureanhydrid

9-Bromoanthracene
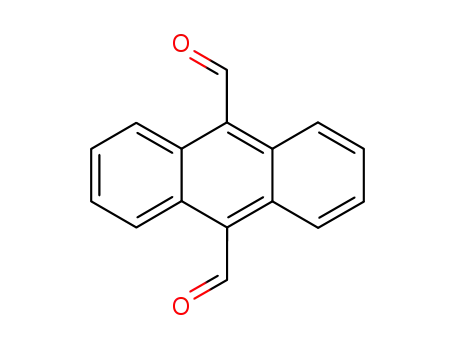
9,10-diformylanthracene