Your Location:Home > Products > Pharmaceutical > Benzoicacid, 2-chloro-5-iodo-


CasNo: 19094-56-5
Molecular Formula: C7H4ClIO2
Appearance: white to light yellow crystal powder
|
19094-56-5 Name |
|
|
Name |
2-Chloro-5-iodobenzoic acid |
|
Synonym |
2-CHLORO-5-IODOBENZOIC ACID;BUTTPARK 100\01-43;2-Chloro-5-iodobenzoic acid, 98+%;SOTA-011;2 - chloro - 5 - iodine benzoic acid;Chloro-5-iodobenzoicA;2-Chloro-5-iodobnezoic Acid;2-choloro-5-iodobenzoicacid |
|
19094-56-5 Chemical & Physical Properties |
|
|
Melting point |
157-161 °C(lit.) |
|
Boiling point |
353.1±27.0 °C at 760 mmHg |
|
Density |
2.1±0.1 g/cm3 |
|
Molecular Formula |
C7H4ClIO2 |
|
Molecular Weight |
282.463 |
|
Flash Point |
167.4±23.7 °C |
|
PSA |
37.30000 |
|
LogP |
3.18 |
|
Exact Mass |
281.894440 |
|
Vapour Pressure |
0.0±0.8 mmHg at 25°C |
|
Index of Refraction |
1.673 |
2-Chloro-5-iodobenzoic acid is white to light yellow crystal powder, which is used as starting material.
InChI:InChI=1/C7H4ClIO2/c8-6-2-1-4(9)3-5(6)7(10)11/h1-3H,(H,10,11)
Sodium-glucose co-transporter (SGLT) inhibitors are a novel class of therapeutic agents for the treatment of type 2 diabetes based on blocking of renal reabsorption of glucose. Dapagliflozin, a C-aryl glucoside, has emerged as a successful drug in the market based on this concept. These results presented herein amply demonstrate the promise of C-benzyl analogues of Dapagliflozin as novel SGLT2 inhibitors for future investigations.
It was synthesized within three steps starting from 2-chloro-5-iodobenzoic acid and substituted benzene. The microtubule destabilizing activities were evaluated in vitro with human liver cancer Huh-7 cell line and human lung cancer A549 cell line. Some of the HPAs were achieved IC50 about 5.0 μM against human liver cancer Huh-7 cells.
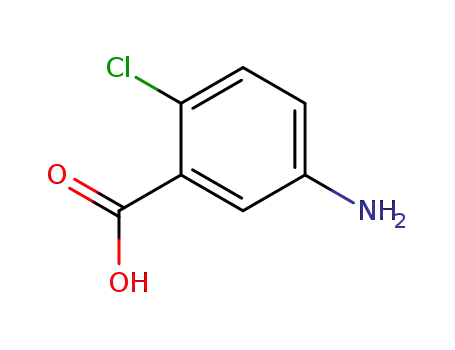
2-chloro-5-aminobenzoic acid

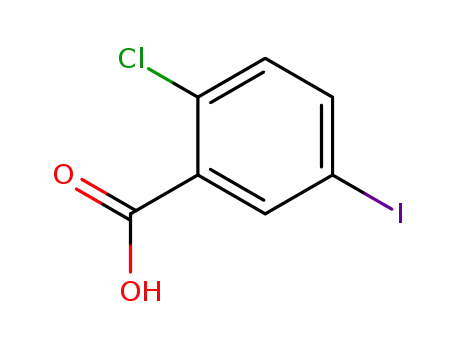
2-chloro-5-iodobenzoic acid
| Conditions | Yield |
|---|---|
|
2-chloro-5-aminobenzoic acid; With sulfuric acid; sodium nitrite; In water; at 0 - 10 ℃;
With urea; potassium iodide; In water; at 0 ℃; for 0.5h;
|
93.7% |
|
With toluene-4-sulfonic acid; sodium iodide; In water; acetonitrile; at 10 - 20 ℃; for 2h; Inert atmosphere;
|
79% |
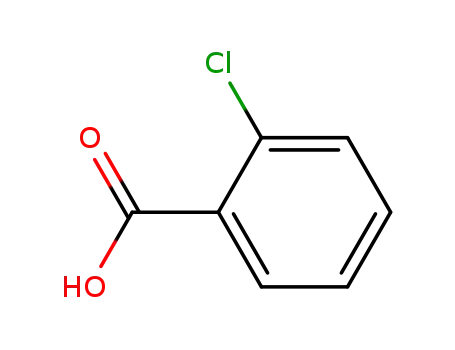
ortho-chlorobenzoic acid


2-chloro-5-iodobenzoic acid
| Conditions | Yield |
|---|---|
|
With potassium iodate; sulfuric acid; iodine; In dichloromethane; at 25 - 30 ℃; for 1h; Reagent/catalyst;
|
69.6% |
|
Multi-step reaction with 3 steps
1.1: nitric acid; sulfuric acid / 2 h / -5 - 5 °C
2.1: iron; ammonium chloride / ethanol; water / 5 h / 78 - 80 °C
3.1: sodium nitrite; sulfuric acid / water / 0 - 10 °C
3.2: 0.5 h / 0 °C
With sulfuric acid; nitric acid; iron; ammonium chloride; sodium nitrite; In ethanol; water;
|

ortho-chlorobenzoic acid

2-chloro-5-aminobenzoic acid
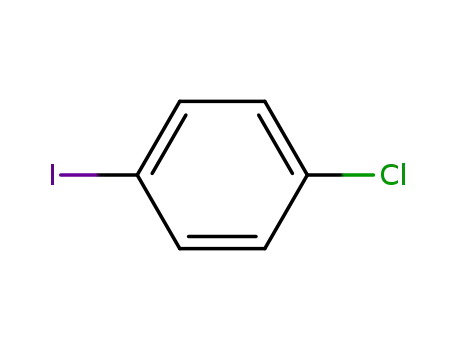
1-Chloro-4-iodobenzene
carbon dioxide
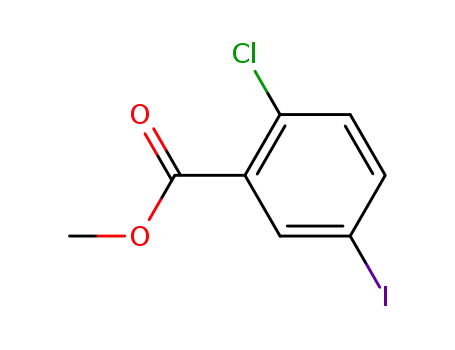
3-carboxymethyl-4-chloro-1-iodobenzene
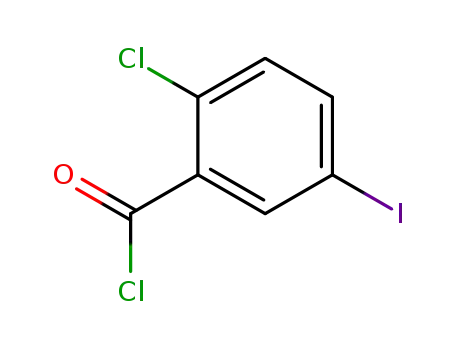
2-chloro-5-iodobenzoylchloride
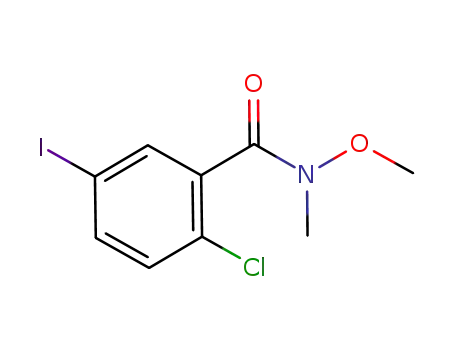
2-chloro-5-iodo-N-methoxy-N-methylbenzamide
2-chloro-5-iodo-benzoic acid 1,1-dimethylethyl ester