Your Location:Home > Products > Fine Chemicals > Phenol, 4-amino-,1-(4-aminobenzoate)


CasNo: 20610-77-9
Molecular Formula: C13H12N2O2
|
20610-77-9 Name |
|
|
Name |
4-Aminobenzoic acid 4-aminophenyl ester |
|
Synonym |
4-Aminobenzoic acid 4-aminophenyl ester;4-Aminophenyl 4-aminobenzoate;APAB;4-AMINOBENZOICACID4-AMINOPHENYLESTER/ETNT;4-AMinophenyl 4-aMinobenzoate(APAB);Phenol, 4-aMino-, 1-(4-aMinobenzoate);Phenol, 4-amino-,4-aminobenzoate (ester) (9CI);4-Aminophenyl4-Aminobenzoate> |
|
20610-77-9 Chemical & Physical Properties |
|
|
Melting point |
179.0 to 183.0 °C |
|
Boiling point |
465.646ºC at 760 mmHg |
|
Density |
1.286 |
|
Molecular Formula |
C13H12N2O2 |
|
Molecular Weight |
228.24700 |
|
Flash Point |
254.224ºC |
|
PSA |
78.34000 |
|
LogP |
3.23260 |
|
Exact Mass |
228.09000 |
|
storage temp. |
Keep in dark place,Sealed in dry,Room Temperature |
4-aminophenyl-4′-aminobenzoate (APAB) is off-white to white powder. Liquid crystalline polymers (LCPs) were synthesised using pyromellitic dianhydride and 4-[(4-aminobenzylidene)amino]aniline or 4-aminophenyl-4-aminobenzoate.
The study of the reaction of 4-(2,3-epoxypropoxy)-4′-ethoxy-biphenyl (1) and of 4-butoxyphenyl-4-(2,3-epoxypropoxy)-benzoate (2) with 4,4′-diaminobiphenyl (3), and 4-aminophenyl-4-aminobenzoate (4) has shown that model compounds are very useful for the understanding and for modelling of the formation of a liquid crystal thermoset.
Seven anisotropic diimines and their corresponding dinuclear complexes of rhenium(i) have been synthesized. In common with related inline ligands which have been synthesized previously, the new diimines were mesomorphic showing smectic C and nematic phases and, in some cases, smectic I and crystal J phases. However, none of the orthometallated, dinuclear complexes showed any liquid crystal phases. The Royal Society of Chemistry 2000.
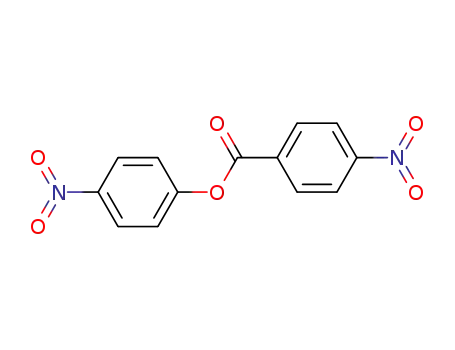
4-nitrophenyl 4-nitrobenzoate

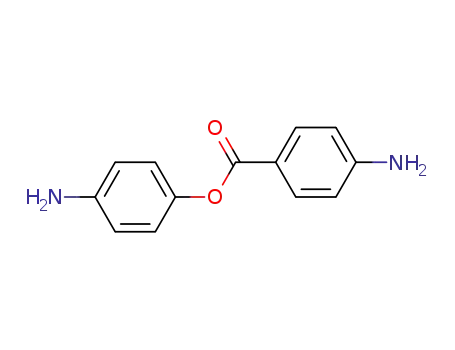
4-aminophenyl 4'-aminobenzoate
| Conditions | Yield |
|---|---|
|
With hydrogen; palladium 10% on activated carbon; In ethyl acetate;
|
99% |
|
With hydrogen; palladium on activated charcoal; In tetrahydrofuran; under 1034.32 Torr;
|
|
|
With palladium on activated charcoal; hydrogen; In ethyl acetate; at 20 ℃; for 16h;
|
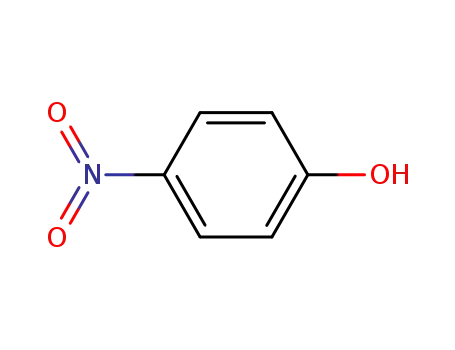
4-nitro-phenol


4-aminophenyl 4'-aminobenzoate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: dicyclohexylcarbodiimide; DMAP / CH2Cl2 / 18 h
2: H2 / palladium on carbon / tetrahydrofuran / 1034.32 Torr
With dmap; hydrogen; dicyclohexyl-carbodiimide; palladium on activated charcoal; In tetrahydrofuran; dichloromethane; 1: Esterification / 2: Catalytic hydrogenation;
|

4-nitrophenyl 4-nitrobenzoate

4-nitro-phenol
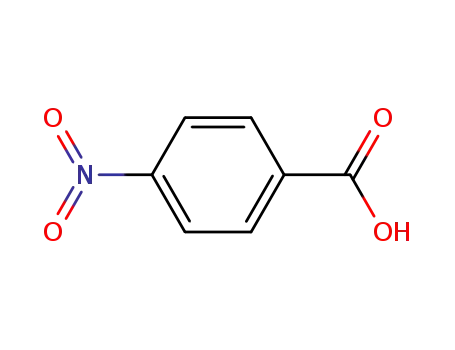
4-nitro-benzoic acid
4-nitro-benzoyl chloride