Your Location:Home > Products > Fine Chemicals > 2-Bromo-1,10-phenanthroline


CasNo: 22426-14-8
Molecular Formula: C12H7 Br N2
|
22426-14-8 Name |
|
|
Name |
2-Bromo-1,10-phenanthroline |
|
Synonym |
2-Bromo-1,10-phenathroline;2-Bromo-1,10-phenanthroline;2-Bromo-1,10-phenant;2-Bromo-1,10-phenthroline;TC-OM-10;1,10-Phenanthroline, 2-bromo- |
|
22426-14-8 Chemical & Physical Properties |
|
|
Melting point |
161.0 to 165.0 °C |
|
Boiling point |
414.3±25.0 °C at 760 mmHg |
|
Density |
1.6±0.1 g/cm3 |
|
Molecular Formula |
C12H7BrN2 |
|
Molecular Weight |
259.101 |
|
Flash Point |
204.3±23.2 °C |
|
PSA |
25.78000 |
|
LogP |
2.62 |
|
Exact Mass |
257.979248 |
|
Vapour Pressure |
0.0±0.9 mmHg at 25°C |
|
Index of Refraction |
1.758 |
Uses
Brominated phenanthroline can be synthesized via a method reported by Baran and coworkers using a two-step protocol through N-Oxide formation using m-CBPA (Aldrich 273031) and bromination using TBAB (Sigma-Aldrich 426288) and p-Toluenesulfonic anhydride (Aldrich 259764).
InChI:InChI=1/C12H7BrN2/c13-10-6-5-9-4-3-8-2-1-7-14-11(8)12(9)15-10/h1-7H
The 2-bromo-1,10-phenanthroline and 1,4-diiodotetrafluorobenzene for crystallization were purchased from J&K Scientific Ltd., and used without further purification. The halogen bond donor 1,4-diiodotetrafluorobenzene (4.02 mg, 0.01 mmol) and the halogen bond acceptor 2-bromo-1,10-phenanthroline (1.87 mg, 0.01 mmol) were dissolved in approximately 10 mL of trichloromethane with gentle stirring at room temperature. It is significant to compare the crystal structure of the cocrystal formed between 2-chloro-1,10-phenanthroline and 1,4-diiodotetrafluorobenzene with the crystal structure of the cocrystal formed between 2-bromo-1,10-phenanthroline and 1,4-diiodotetrafluorobenzene.
A mild method for the regioselective C2-bromination of fused azine N-oxides is presented, employing tosic anhydride as the activator and tetra-n-butylammonium bromide as the nucleophilic bromide source. The C2-brominated compounds are produced in moderate to excellent yields and with excellent regioselectivity in most cases. The potential extension of this method to other halogens, effecting C2-chlorination with Ts2O/TBACl is also presented. Finally, this method could be incorporated into a viable one-pot oxidation/bromination process, using methyltrioxorhenium/urea hydropgen peroxide as the oxidant.
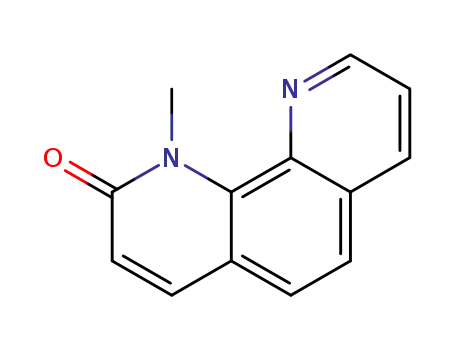
1-methyl-1,10-phenanthrolin-2(1H)-one

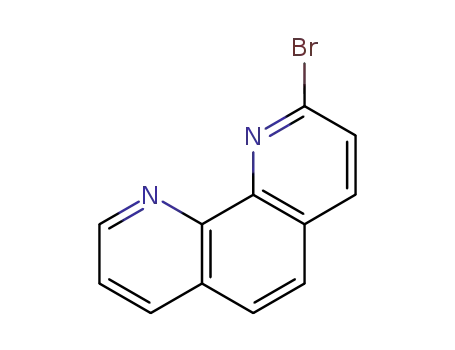
2--bromo-1,10--phenanthroline
| Conditions | Yield |
|---|---|
|
1-methyl-1,10-phenanthrolin-2(1H)-one; With phosphorus pentabromide; phosphorus(V) oxybromide; at 20 - 80 ℃; Inert atmosphere;
With ammonia; In water; Alkaline conditions;
|
94% |
|
1-methyl-1,10-phenanthrolin-2(1H)-one; With phosphorus pentabromide; phosphorus(V) oxybromide; at 80 ℃; for 6h; Inert atmosphere; Cooling with ice;
With ammonia; In water; Cooling with ice;
|
94% |
|
With bromine; triphenylphosphine; In acetonitrile; at 0 ℃; for 18h; Reflux;
|
55% |
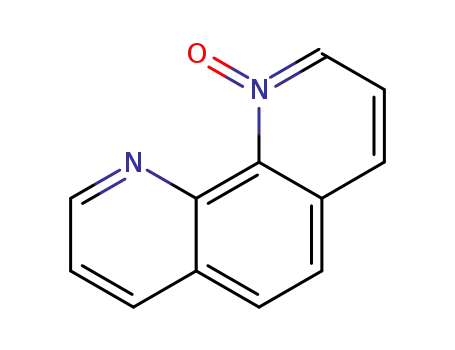
1,10-phenanthroline N-oxide


2--bromo-1,10--phenanthroline
| Conditions | Yield |
|---|---|
|
1,10-phenanthroline N-oxide; With tetrabutylammomium bromide; In dichloromethane; at 20 ℃; for 0.166667h; Inert atmosphere; Molecular sieve;
With p-toluenesulfonylanhydride; In dichloromethane; at 20 ℃; regioselective reaction; Inert atmosphere;
|
70% |
|
With N,N-dimethyl-formamide; phosphorus(V) oxybromide; In dichloromethane; at 0 - 25 ℃; regioselective reaction; Inert atmosphere;
|
52% |
|
1,10-phenanthroline N-oxide; With tetrabutyl ammonium fluoride; In dichloromethane; at 20 ℃; for 0.166667h; Molecular sieve;
With p-toluenesulfonylanhydride; In dichloromethane; at 20 ℃;
|
48% |
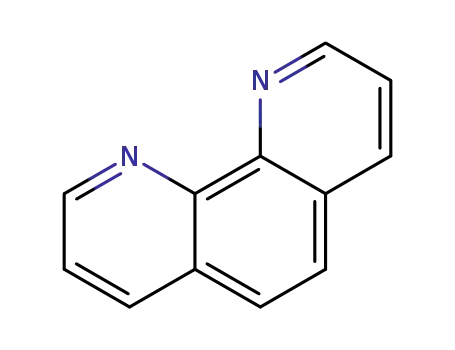
1,10-Phenanthroline

1-methyl-1,10-phenanthrolin-2(1H)-one
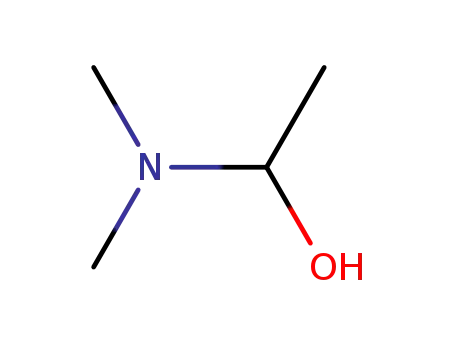
1-Dimethylamino-ethanol
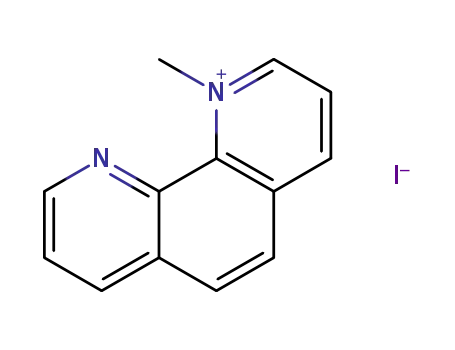
1-methyl-1,10-phenanthrolinium iodide
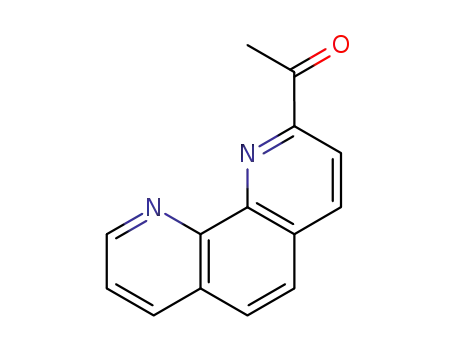
2-acetyl-1,10-phenanthroline
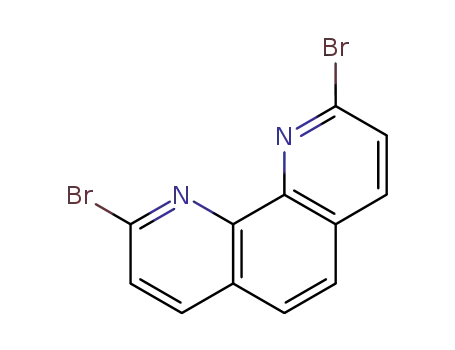
2,9-dibromo-1,10-phenanthroline
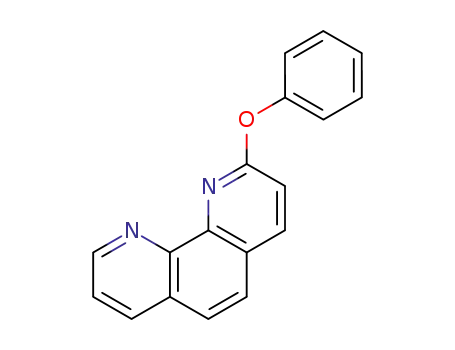
2-phenoxy-1,10-phenanthroline
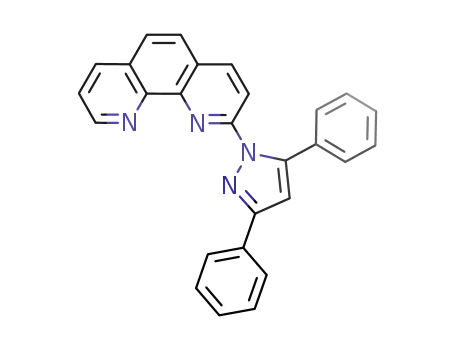
2-(3,5-diphenyl-1H-pyrazol-1-yl)-1,10-phenanthroline