Your Location:Home > Products > Cannabis > 2,4-DIHYDROXY-6-PENTYL-BENZALDEHYDE

CasNo: 855875-40-0
|
855875-40-0 Name |
|
|
Name |
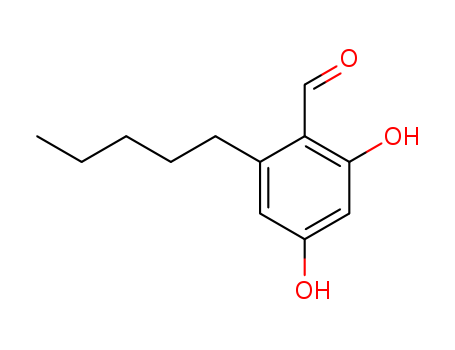
2,4-DIHYDROXY-6-PENTYL-BENZALDEHYDE |
|
Synonym |
2,4-Dihydroxy-6-pentyl-benzaldehyde;Benzaldehyde, 2,4-dihydroxy-6-pentyl- |
|
855875-40-0 Chemical & Physical Properties |
|
|
Form |
Oil |
|
Color |
Dark |
|
Molecular Formula |
C12H16O3 |
|
Molecular Weight |
208.25400 |
|
PSA |
57.53000 |
|
LogP |
2.64300 |
|
Exact Mass |
208.11000 |
|
solubility |
Methanol |
The present invention can synthesize lobaric acid and four analogues thereof, which are five phenolic lichen metabolites isolated from an extract of the Antarctic lichen Stereocaulon alpinum and selectively inhibit PTP1B, by a simple, economic and efficient chemical synthesis method.
The present invention provides simple synthetic routes for the preparation of cannabinoid compounds such as CBD, CBDV, THC, THCV, CBN, HU-308, CBG, CBC, and derivatives thereof, which are stereoselective and provide the desired cannabinoid compound in high yield.
The first total syntheses of the natural products lobaric acid (1) and its derivatives isolated from the Antarctic lichen Stereocaulon alpinum are reported in this study. Lobarin (3), with a pseudodepsidone structure, was synthesized first in 11 steps by utilizing an Ullmann aryl ether coupling reaction, and lobaric acid was synthesized in an additional three steps by a seven-membered lactonization reaction. Various derivatives were also obtained from the prepared lobaric acid, and the synthetic compounds exhibited significant PTP1B inhibitory activities.
Naturally occurring anziaic acid has very recently been reported as a topoisomerase I inhibitor with antibacterial activity. Herein total synthesis of anziaic acid and its structural analogues is described and the preliminary structure-activity relationship (SAR) has been developed based on topoisomerase inhibition and whole cell antibacterial activity.
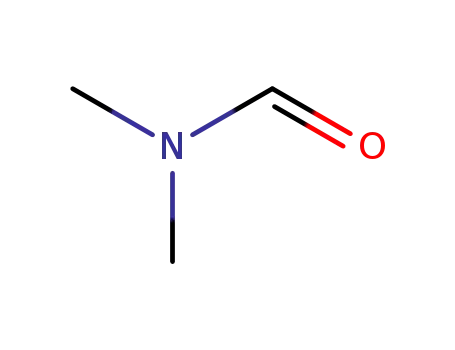
N,N-dimethyl-formamide

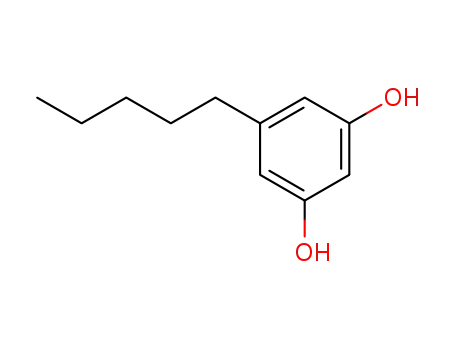
Olivetol

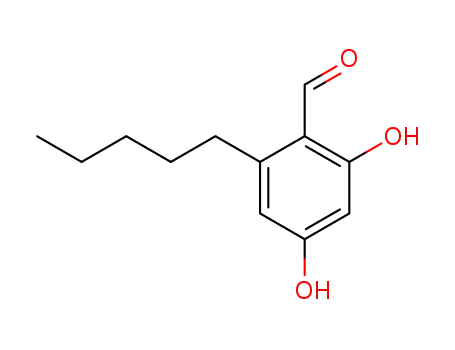
2,4-dihydroxyl-6-pentylbenzaldehyde
| Conditions | Yield |
|---|---|
|
With trichlorophosphate; at 0 - 20 ℃; for 8.5h; regioselective reaction; Inert atmosphere;
|
90% |
|
With trichlorophosphate; at 0 - 20 ℃; for 8.5h; Inert atmosphere;
|
90% |
|
With trichlorophosphate; at 0 - 20 ℃; for 18h;
|
56% |
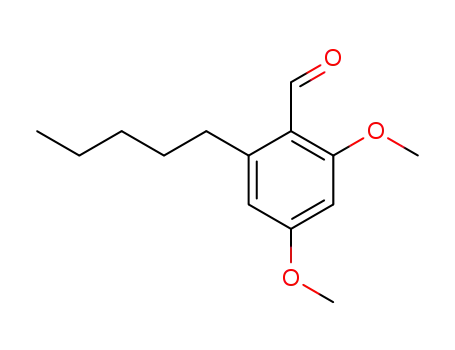
2,4-dimethoxy-6-pentyl-benzaldehyde


2,4-dihydroxyl-6-pentylbenzaldehyde
| Conditions | Yield |
|---|---|
|
With aluminum (III) chloride; In dichloromethane; at 20 - 40 ℃;
|
30 g |

hydrogen cyanide

Olivetol
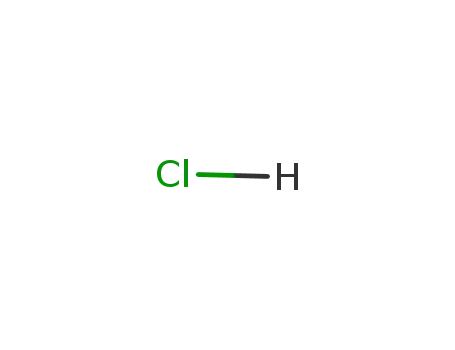
hydrogenchloride
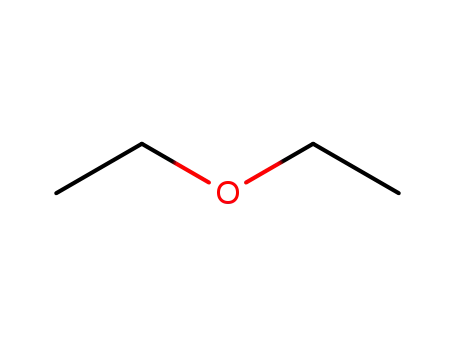
diethyl ether
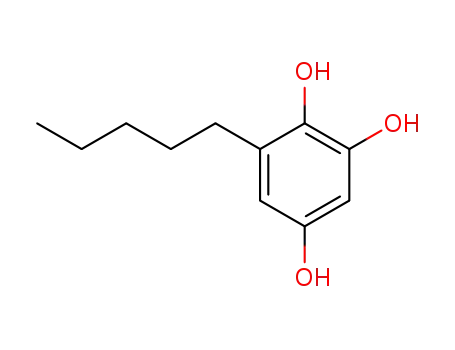
6-(n-pentyl)-1,2,4-trihydroxybenzene
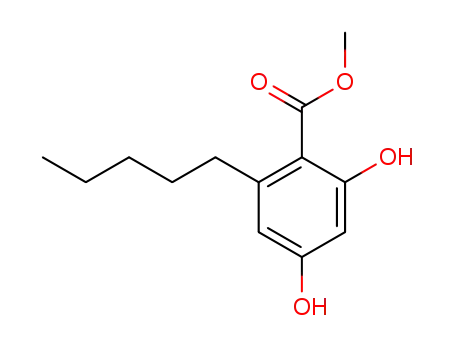
methyl 2,6-dihydroxy-6-pentylbenzoate
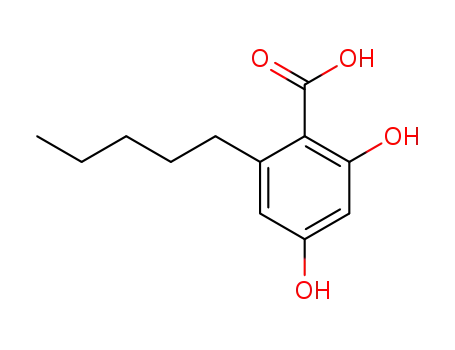
2,4-dihydroxyl-6-pentylbenzoic acid
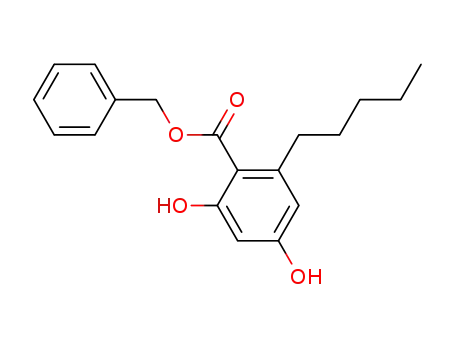
benzyl 2,4-dihydroxy-6-pentylbenzoate