Your Location:Home > Products > Fine Chemicals > 4'-Hydroxyacetophenone
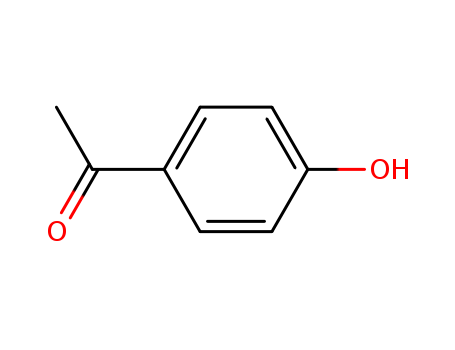

CasNo: 99-93-4
Molecular Formula: C8H8O2
Appearance: almost white to beige crystalline powder
|
99-93-4 Name |
|
|
Name |
4'-Hydroxyacetophenone |
|
Synonym |
1-(4-hydroxyphenyl)-ethanon;4’-hydroxy-acetophenon;4-Hydroksyacetofenol;Acetophenone, p-hydroxy-;Ethanone, 1-(4-hydroxyphenyl)-;Hydroxyacetophenone, para;HYDROXYACETOPHENONE-4;HYDROXYBENZOPHENONE-4 |
|
99-93-4 Biological Activity |
|
|
Related Catalog |
Research Areas >> Infection Signaling Pathways >> Others >> Others Research Areas >> Inflammation/Immunology |
|
99-93-4 Chemical & Physical Properties |
|
|
Melting point |
132-135 °C(lit.) |
|
Boiling point |
313.0±0.0 °C at 760 mmHg |
|
Density |
1.1±0.1 g/cm3 |
|
Molecular Formula |
C8H8O2 |
|
Molecular Weight |
136.148 |
|
Flash Point |
121.2±12.4 °C |
|
PSA |
37.30000 |
|
LogP |
1.42 |
|
Exact Mass |
136.052429 |
|
Vapour Pressure |
0.0±0.7 mmHg at 25°C |
|
Index of Refraction |
1.552 |
|
Storage condition |
Refrigerator |
|
Water Solubility |
10 g/L (22 ºC) |
4'-hydroxyacetophenone (4-HAP) is a monohydroxyacetophenone carrying a hydroxy substituent at position 4'. It has a role as a plant metabolite, a fungal metabolite and a mouse metabolite. It has a good effect on yellow eyes caused by diseases such as hepatitis, and also has a good auxiliary effect on yellow eyes caused by various reasons. According to the classification provided by companies to ECHA in REACH registrations this substance causes serious eye irritation and is harmful to aquatic life with long lasting effects.
Definition
ChEBI: A monohydroxyacetophenone carrying a hydroxy substituent at position 4'.
IUPAC Standard InChI: InChI=1S/C11H16O2Si/c1-9(12)10-5-7-11(8-6-10)13-14(2,3)4/h5-8H,1-4H3
InChI version 1.06 IUPAC Standard
InChIKey: HVSXQEUGLVLBLR-UHFFFAOYSA-N
The highest activities of HAPMOPf was 1.47 U·mg−1 toward 4-hydroxyacetophenone among tested ketone and thioether substrates. Furthermore, over 95% enantioselectivity was determined toward methyl phenyl sulfides with ortho, meta, and para-substituents (Cl, Br, CHO, NH2).
Herein, we report the first decarboxylative hydroxylation to synthesize phenols from benzoic acids at 35 °C via photoinduced ligand-to-metal charge transfer (LMCT)-enabled radical decarboxylative carbometalation. The aromatic decarboxylative hydroxylation is synthetically promising due to its mild conditions, broad substrate scope, and late-stage applications.
We believe thatAzaB5is promising as a powerful tool to rapidly delineate a broad range of malignancies and assist surgical tumour resection.
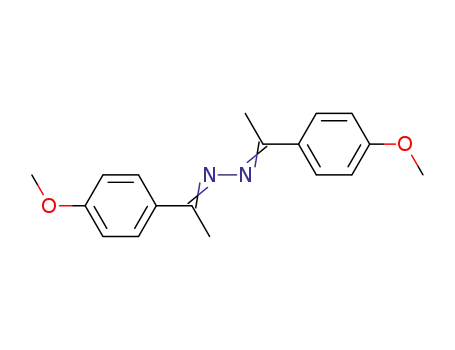
1,2-bis(1-(4-methoxyphenyl)ethylidene)hydrazine

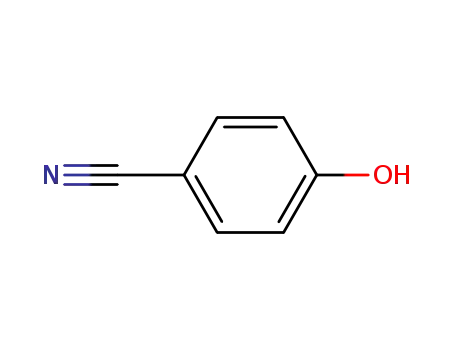
4-cyanophenol

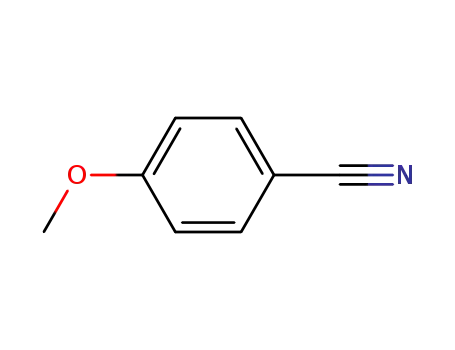
4-methoxybenzonitrile

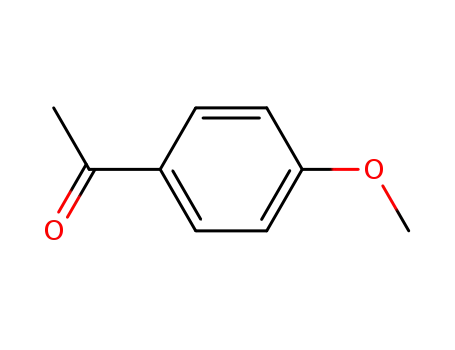
1-(4-methoxyphenyl)ethanone

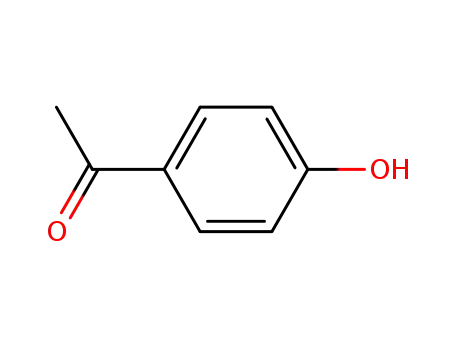
4-Hydroxyacetophenone

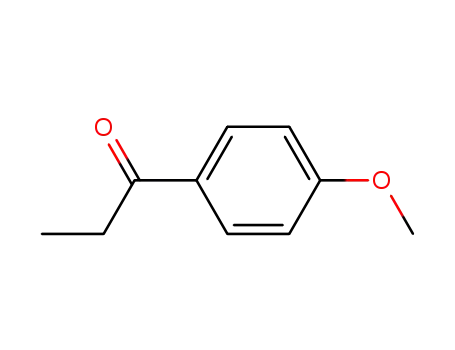
4-Methoxypropiophenone

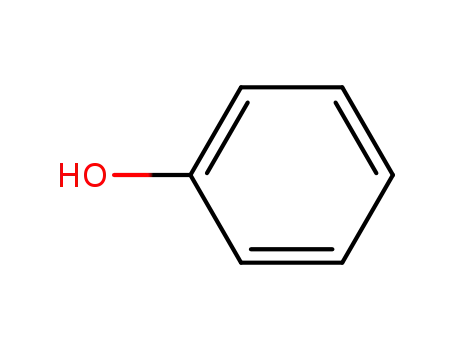
phenol
| Conditions | Yield |
|---|---|
|
at 20 - 350 ℃; Product distribution;
|
45.7 % Chromat. 1.5 % Chromat. 0.8 % Chromat. 20.3 % Chromat. 1.7 % Chromat. 1.1 % Chromat. |
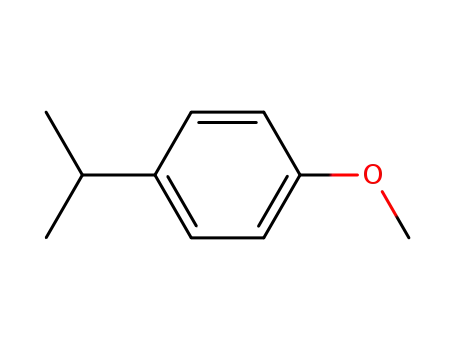
1-methoxy-4-(1-methylethyl)benzene

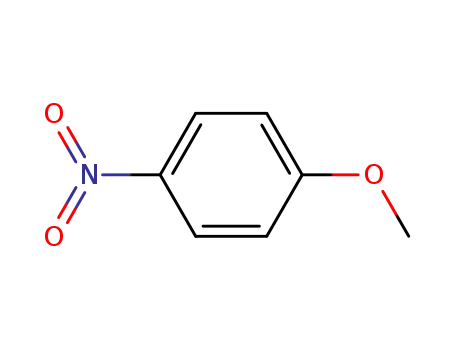
para-methoxynitrobenzene

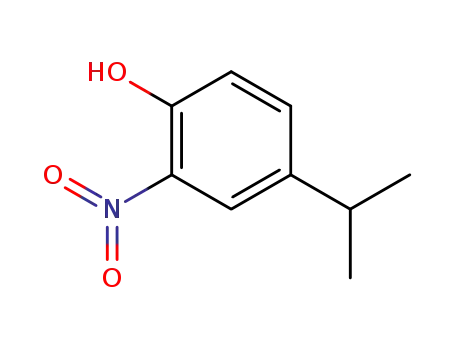
4-isopropyl-2-nitrophenol

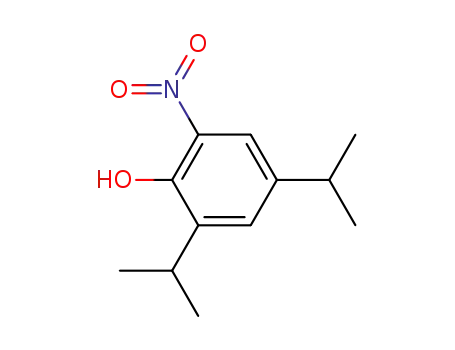
4,6-di-isopropyl-2-nitrophenol

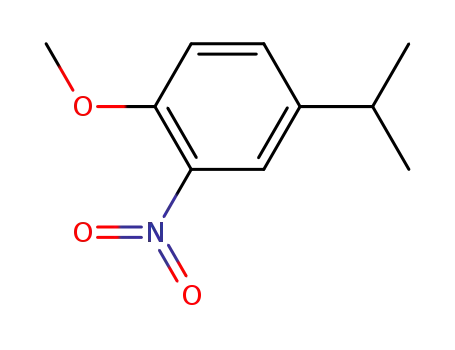
4-isopropyl-2-nitro-anisole


1-(4-methoxyphenyl)ethanone


4-Hydroxyacetophenone
| Conditions | Yield |
|---|---|
|
With nitric acid; 4-aminobenzene sulfonic acid; In sulfuric acid; at 25 ℃; Product distribution; Rate constant; various concentrations and molar ratios of HNO3 and H2SO4;
|
vitexin
1,1,1-trichloroethane
sodium phenoxide
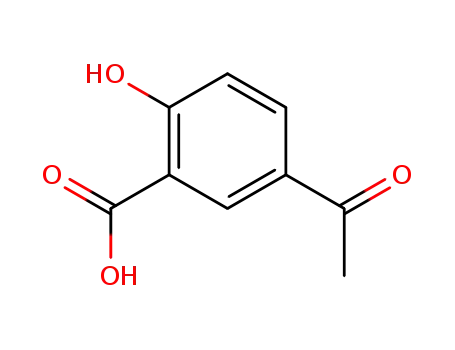
5-acetylsalicylic acid
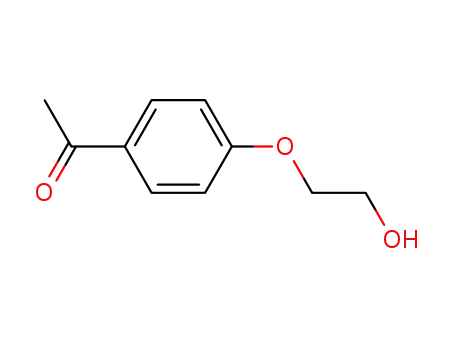
1-(4-(2-hydroxyethoxy)phenyl)ethanone
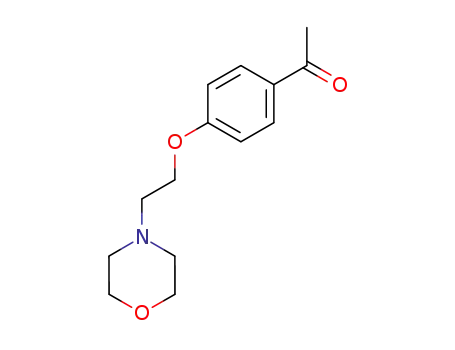
1-(4-(2-morpholinoethoxy)phenyl)ethanone
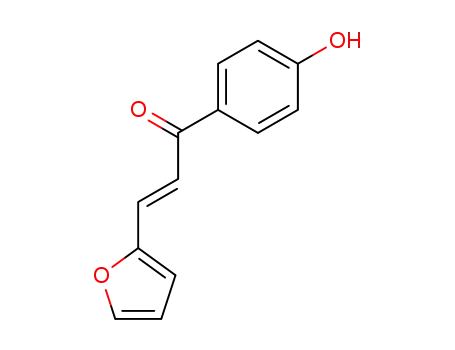
(2E)-3-(furan-2-il)-1-(4-hidroxifenil)prop-2-en-1-ona
1-(4-(2-(piperidin-1-yl)ethoxy)phenyl)ethanone